Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Effective, non-invasive disease activity and treatment response assessments are needed for patients with systemic lupus erythematosus (SLE), especially if associated with kidney disease, i.e. lupus nephritis (LN). The treatment goal of LN is to achieve complete renal remission (CRR). Proteinuria of >0.5 g/day can prompt a kidney biopsy to diagnose LN. The Renal Activity Index for Lupus (RAIL) measures the degree of kidney inflammation. The RAIL-score is calculated from the creatinine-adjusted RAIL biomarkers (neutrophil gelatinase-associated lipocalin [NGAL], kidney injury molecule-1 [KIM-1], monocyte chemoattractant protein-1 [MCP-1], adiponectin, hemopexin, ceruloplasmin) and higher scores indicate higher kidney inflammation.1 This study evaluated 1) the role of RAIL to distinguish CRR status and identify the cut-point for RAIL; 2) the role of RAIL in predicting change in CRR status over time.
Methods: Urine samples collected from 69 SLE adult patients with and without LN were studied longitudinally at enrollment into the GLADEL cohort (T0), at 6 months (T1) and 12 months (T2). Absolute scores and changes in RAIL-scores over time were assessed for presence of CRR status (proteinuria of < 0.5 g/day) by logistical regression models. The Youden Index optimal cut-points on the receiver operating characteristic (ROC) curves were calculated.
Results: For 186 visits from 51 (74%; 91% female) patients with LN and 18 (26%) patients without LN diagnosis, patient characteristics and disease courses are shown in Table 1. RAIL-scores were correlated with renal-SLEDAI (r=0.46; p< 0.0001) and proteinuria (r=0.37; p=0.002). Considering all visits (CRR present/absent=146/40), mean (SD) RAIL-scores with CRR-status were 1.17 (1.53) lower than without CRR (p=0.023; area under the ROC curve =0.73), as shown in Figure 1, RAIL scores of no more than 3.4 identified CRR-status with 78% specificity (Positive Predictive Value [PPV]=0.77, sensitivity=62). Patients who newly achieved CRR-status at the next visit had a mean (SD) RAIL-score decrease of 1.0 (1.524) since the last visit (Percentage Variance Value [PVV]=82%, p=0.0024) and a further decrease of >0.57 of achieving CRR at the next visit.
Conclusion: RAIL-scores are significantly lower with CRR-status in LN and decreases of 1.0 between visits >0.57 or larger may predict future CRR achievement.
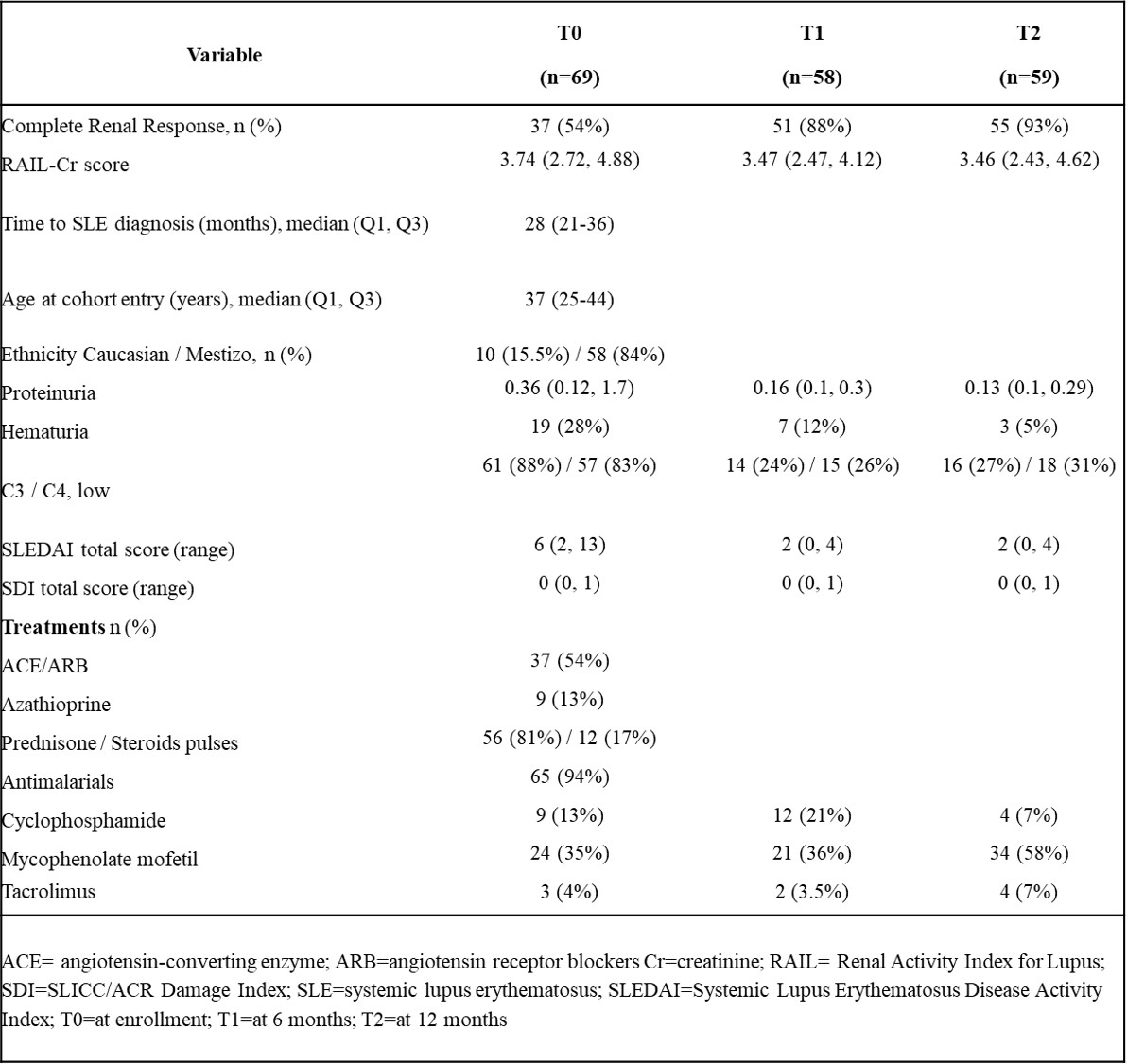 Table 1. Patient characteristics & RAIL-scores over time
Table 1. Patient characteristics & RAIL-scores over time
To cite this abstract in AMA style:
Pons-Estel G, Quintana R, Nieto R, Brunner H, Scolnik M, Funes Soaje C, Alba P, Saurit V, Garcia M, BERBOTTO G, Bellomio I, Kerzberg M, Gomez G, Pisoni C, Juarez V, Malvar A, Silva N, MONTICIELO O, Mariz H, Ribeiro F, Borba E, Bonfa E, Torres dos Reis-Neto E, Guerra Herrera I, Massardo M, Aroca-Martínez G, Gómez Escorcia L, Cañas C, Quintana-Lopez G, Toro-Gutierrez C, Moreno Alvarez M, SAAVEDRA M, Portela Hernández M, Fragoso-Loyo H, Silveira L, García-De la Torre I, Abud-Mendoza C, Esquivel Valerio J, Acosta M, Paats A, Mora-Trujillo C, Ugarte-Gil M, Calvo A, Muñoz-Louis R, Rebella M, Danza A, Zazzetti F, Orillion A, Sbarigia U, Pons-Estel B. The Renal Activity Index for Lupus Identifies and Predicts Complete Renal Remission or Absence of Kidney Involvement in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-renal-activity-index-for-lupus-identifies-and-predicts-complete-renal-remission-or-absence-of-kidney-involvement-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-renal-activity-index-for-lupus-identifies-and-predicts-complete-renal-remission-or-absence-of-kidney-involvement-in-systemic-lupus-erythematosus/

.jpg)