Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immunogenicity of pegloticase, a PEGylated uricase, can limit a sustained urate-lowering response and increase risk for infusion reactions (IRs).1 The MIRROR RCT trial demonstrated that methotrexate (MTX), when administered as co-therapy to pegloticase, increased treatment response rate (MTX vs. placebo [PBO] co-therapy: 71% vs. 39% during Month 6), decreased IR risk (4% vs. 31% thru Month 6), and decreased development of de novo anti-drug antibodies (ADAs; MTX vs. PBO: 23% vs. 50% thru Month 6; lower titers in MTX group).2 Here, we explored the relationship between ADAs and loss of SU-lowering response (LOR) and IR.
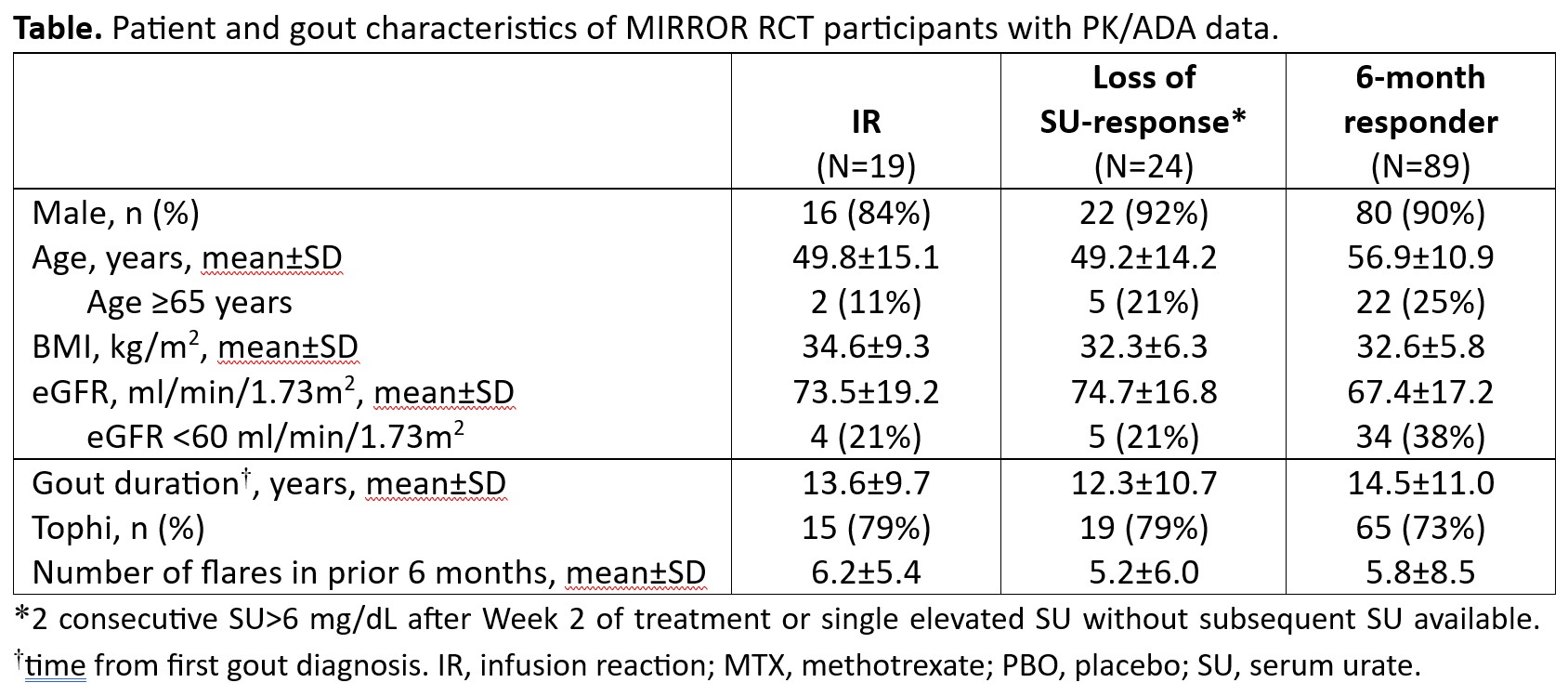
Methods: Patients with uncontrolled gout (SU≥7 mg/dL, oral ULT inefficacy/intolerance and gout features [≥1 tophus, ≥2 flares/year, and/or gouty arthropathy) were administered up to 52 weeks of pegloticase (8 mg every 2 weeks; first infusion Day 1) with either oral MTX (15 mg/week) or PBO co-therapy (4-week MTX/PBO Run-in). All patients received both flare prophylaxis (≥1 week before Day 1) and pre-infusion prophylaxis. Blood samples for ADA titer measurement (IgG anti-PEG, anti-uricase; pre-infusion, determined by ELISA) and pharmacokinetics (PK, pre- and post-infusion) were collected at Day 1 and pre-specified timepoints. In this post hoc analysis, patients were classified into those with IR, LOR (2 consecutive SU< 6 mg/dL after Week 2 or single elevated SU without subsequent SU available), and SU-lowering response (RESP; SU < 6 mg/dL for ≥80% during Month 6). Subjects with IR were classified as IR, regardless of response/LOR status. ADA data from patients with measurable ADA titers were included regardless of treatment status. PK measurements were only performed in subjects on treatment (includes the 2 weeks following last pegloticase infusion).
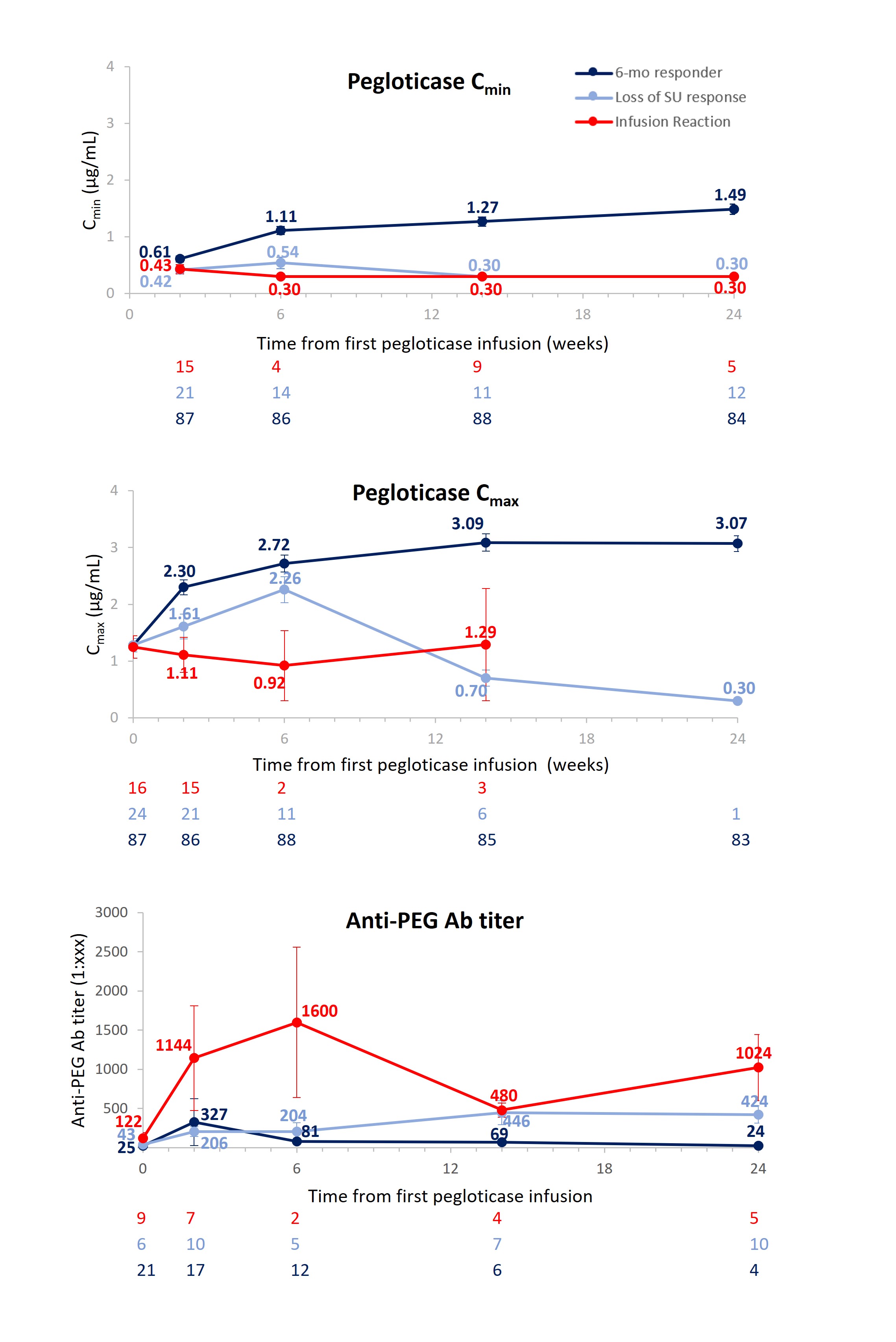
Results: Baseline characteristics were similar among IR (N=19), LOR (N=24), and RESP (N=89) groups (Table). Before pegloticase exposure, 9/19 (47%) IR, 6/24 (25%) LOR, and 21/89 (24%) RESP had measurable IgG anti-PEG antibody (Ab) titers. At all post-Day 1 measured time points, mean IgG anti-PEG Ab titer was highest in the IR group and lowest in the RESP group; correspondingly, mean peak (Cmax) and trough (Cmin) pegloticase concentrations were lowest in the IR group and highest in the RESP group (Figure). IgG anti-uricase Ab development was infrequent in all groups, with only low titers observed.
Conclusion: These data further investigate the relationship between anti-PEG Abs, LOR, and IR in patients treated with pegloticase. Consistent with prior study,1 anti-uricase Abs did not seem to influence pegloticase safety or efficacy. These results further support a negative correlation between pegloticase concentrations and anti-PEG Ab titers, as well as elevated titers, in those experiencing LOR or an IR. It is notable that patients experiencing an IR during pegloticase infusion, on average, had a higher pre-formed anti-PEG titer, earlier development of de novo anti-PEG Abs, and a higher anti-PEG Ab titer following pegloticase exposure than those experiencing LOR.
References:
1. Lipsky PE, et al. Arthritis Res Ther. 2014;16:R60
2. Botson JK, et al. Arthritis Rheumatol. 2023;75:293-304
To cite this abstract in AMA style:
Chabra S, Mohammad A, Obermeyer K, Song Y, Padnick-Silver L, LaMoreaux B, Lipsky P. The Relationship Between Anti-drug Antibodies, Infusion Reactions, and Loss of Urate-lowering Response in Patients with Uncontrolled Gout Treated with Pegloticase [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-relationship-between-anti-drug-antibodies-infusion-reactions-and-loss-of-urate-lowering-response-in-patients-with-uncontrolled-gout-treated-with-pegloticase/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-relationship-between-anti-drug-antibodies-infusion-reactions-and-loss-of-urate-lowering-response-in-patients-with-uncontrolled-gout-treated-with-pegloticase/