Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The purpose of this investigation was to characterize the relationship between systemic exposure of abatacept and measures of target engagement to further support dosing recommendations for abatacept.
Methods: The ALLOW study was a multinational, multicenter, randomized, double-blind, withdrawal study in patients with mild to moderate RA on a stable background of methotrexate to evaluate the immunogenicity and safety of subcutaneous (SC) abatacept. A key exploratory objective in this study was assessing the extent of CD86 receptor saturation in RA subjects following 125 mg weekly SC abatacept during lead-in, withdrawal, and re-introduction periods. The time course of abatacept concentrations as well as total and free CD86 expression levels on the surface of peripheral blood monocytes were measured upon initiation of abatacept as well as during the withdrawal and re-introduction phases using validated assays and was described by an extended linear model. Percentage of CD86 Receptor Occupancy (RO) was derived from the free CD86 expression levels at baseline and post-treatment. The relationship between abatacept concentrations and percentage of CD86 RO was characterized by a Sigmoidal Emax Model.
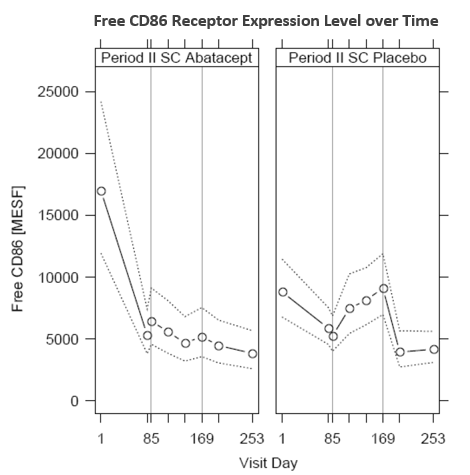
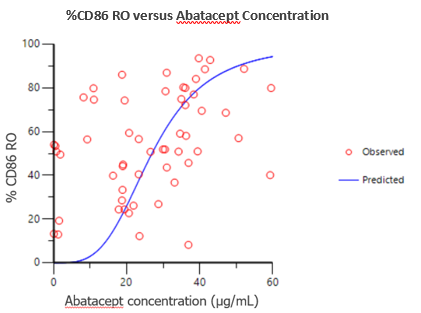
Results: Steady-state abatacept trough concentrations were achieved by Day 57 and were consistent from Days 57 to the end of the lead-in period/beginning of the withdrawal period (Day 85). On Day 85, mean free CD86 expression levels were comparable between the patients randomized to the SC abatacept and SC placebo groups for the withdrawal period. Upon withdrawal of abatacept therapy on Day 85, mean abatacept trough concentrations declined and mean free CD86 expression levels increased over time to baseline levels by the end of the withdrawal period on Day 169 (Figure 1, right panel). For patients who continued to receive SC abatacept, mean steady-state trough abatacept concentrations remained consistent while mean free CD86 expression levels incrementally decreased over time (Figure 1, left panel). Binding of abatacept to the CD86 receptor appears to be concentration dependent and was well described by a Sigmoidal Emax model with an EC50 (CV%) of 26.9 µg/mL (21.1%) (Figure 2). Administration of the approved SC dosing regimen results in steady state trough concentrations of approximately 25-34 µg/mL1-4. Overall, the CD86 binding characteristics are in good agreement with previous exposure-response analyses, where near maximal efficacy (i.e. DAS28 score and ACR20/50/70) is driven by steady state trough concentrations of 10 µg/mL or higher, which is achievable for the approved SC dosing abatacept regimen5.
Conclusion: Establishing PK/RO relationships associated with patient outcomes enables guidance of dose administration for SC abatacept in RA.
To cite this abstract in AMA style:
Abelian G, Gao S, Gandhi Y, Vakkalagadda B, Perera V, Murthy B. The Relationship Between Abatacept Exposure and CD86 Receptor Occupancy in Rheumatoid Arthritis Patients Following Subcutaneous Administration and Its Association to Patient Outcomes [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-relationship-between-abatacept-exposure-and-cd86-receptor-occupancy-in-rheumatoid-arthritis-patients-following-subcutaneous-administration-and-its-association-to-patient-outcomes/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-relationship-between-abatacept-exposure-and-cd86-receptor-occupancy-in-rheumatoid-arthritis-patients-following-subcutaneous-administration-and-its-association-to-patient-outcomes/