Session Information
Date: Sunday, November 12, 2023
Title: (0543–0581) SLE – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with active SLE have periods of uncontrolled disease activity that are associated with a greater risk for developing irreversible organ damage. Current standard of care (SoC) testing does not adequately monitor disease activity, and there is a lack of widespread real-world application of these tests, making it difficult to provide individualized care. To help address this, the lupus activity monitoring panel, comprising a blood test, is designed to assess SLE disease activity by measuring SLE-related biomarkers more comprehensively than SoC tests (see Table 1 footnote). This observational, retrospective cohort study (GSK Study 217538) describes patient and provider characteristics of those who received the lupus activity monitoring panel test and those who received SoC tests.
Methods: Adults with an SLE diagnosis who received at least two lupus activity monitoring panel tests (cases) or at least one set of SoC tests (C3/C4 and anti-dsDNA; controls) between July 1, 2016, and December 31, 2021, were identified in the United Rheumatology-Normal Integrated Community Evidence repository (cases may have also received SoC tests). Eligible patients were linked to administrative medical and pharmacy claims data from Medicare, managed Medicaid and commercial plans. Patients had continuous enrollment for 6 months pre-index (index=first recorded panel/SoC test) and 6 months post index. For cases and controls, patient and provider characteristics were assessed descriptively as mean and standard deviations (SD) for continuous variables and frequencies and proportions for categorical variables.
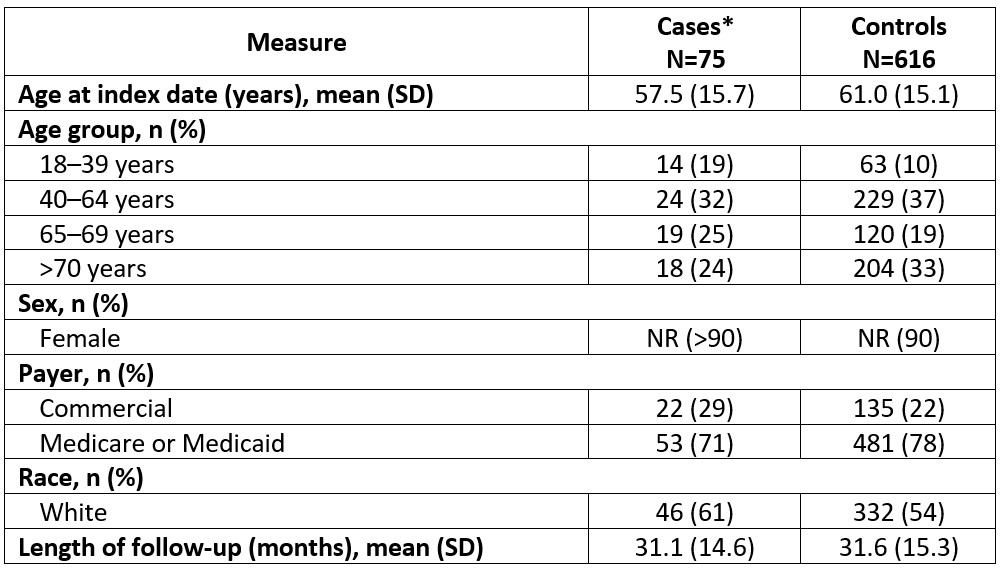
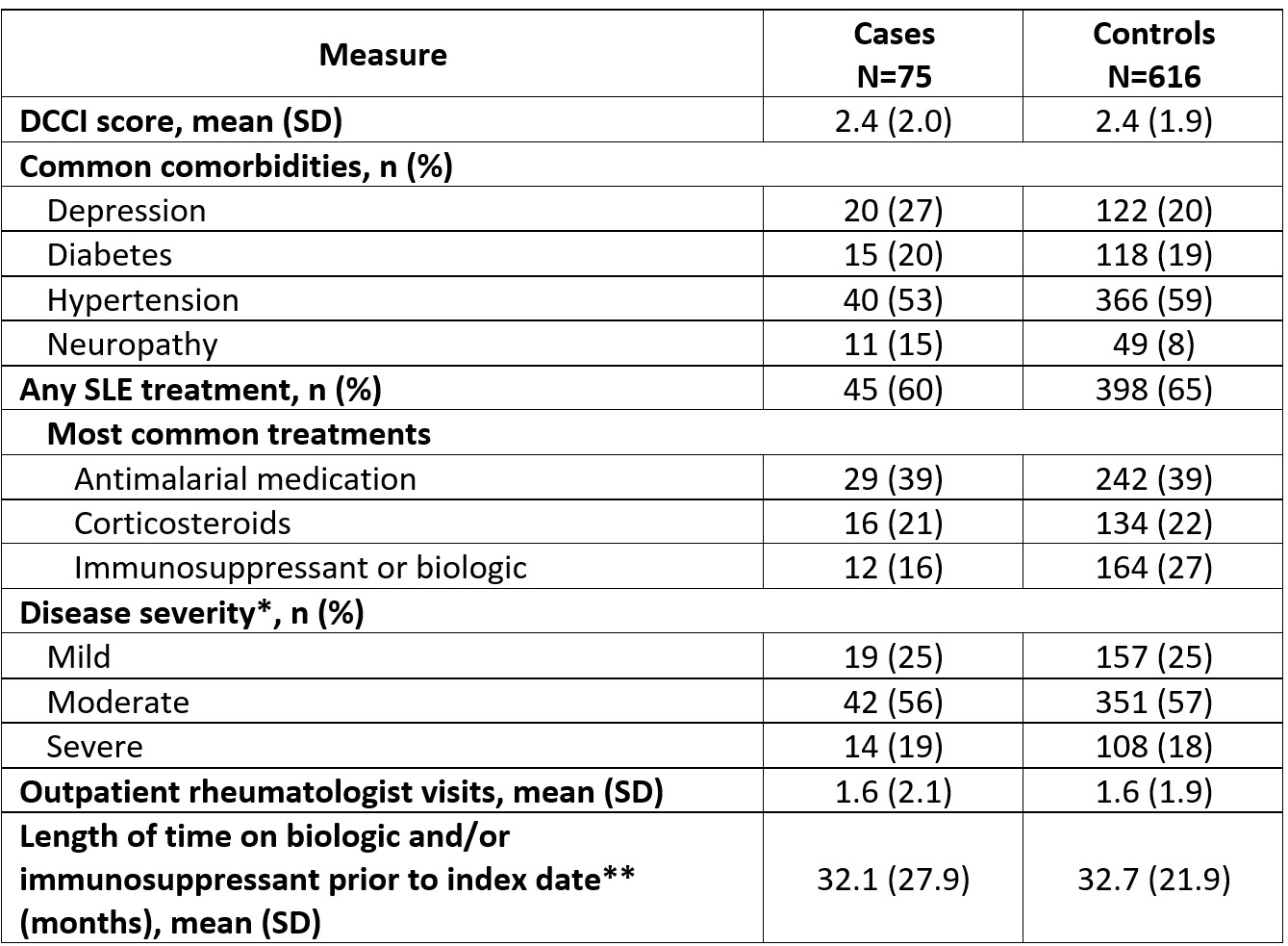
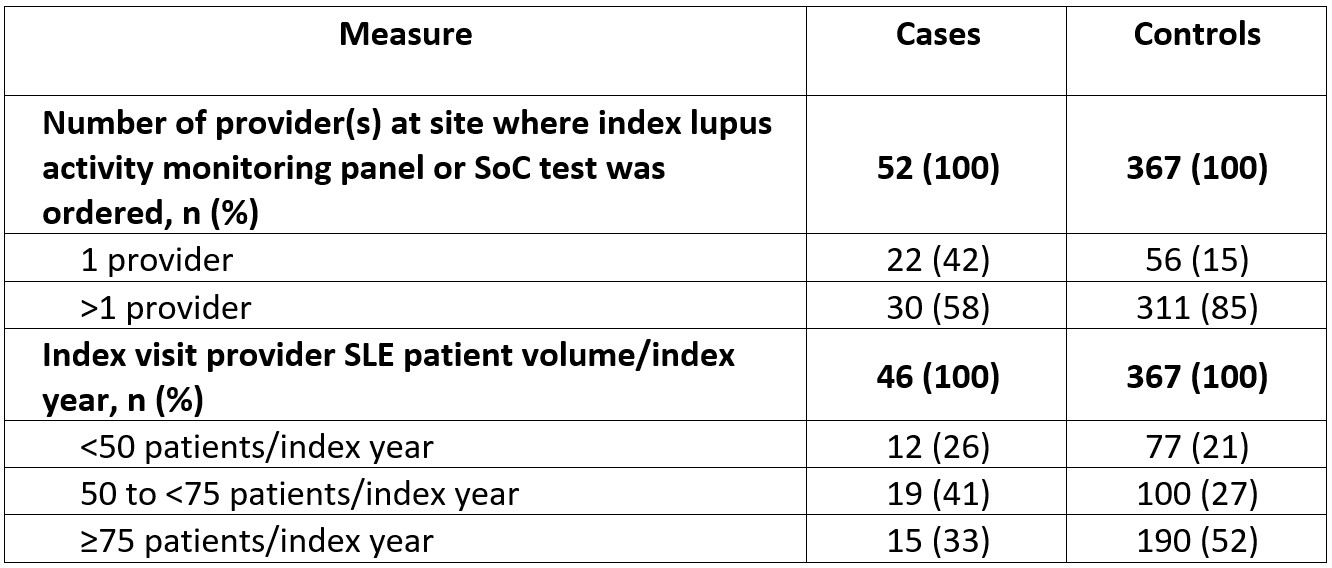
Results: The mean (SD) age for cases (N=75) and controls (N=616) was 57.5 (15.7) and 61.0 (15.1) years, 61% and 54% were white, and 71% and 78% had Medicare or Medicaid insurance, respectively; ≥90% of patients were female (Table 1). Both groups had mean baseline Deyo-Charlson Comorbidity Index scores of 2.4; the most common comorbidities were hypertension (cases, 53%; controls, 59%) and depression (cases, 27%; controls, 20%; Table 2). Approximately half of patients had moderate SLE, and most received ≥1 SLE therapy (cases, 60%; controls, 65%) with antimalarials most commonly prescribed. The lupus activity monitoring panel or SoC index test was ordered at a clinic with only 1 provider for 42% of cases compared with 15% of controls (Table 3). The lupus activity monitoring panel was most frequently used by providers seeing 50 to < 75 patients with SLE/index year, whereas SoC testing was most common in larger practices (Table 3).
Conclusion: This is the first real-world study to describe use of the lupus activity monitoring panel by community-based rheumatologists and offers initial insights into the heterogeneity of this population. There is a need to further understand the lupus activity monitoring panel use in the context of SLE care management and to educate providers on appropriate use of the unique tests included in the lupus activity monitoring panel.
Funding: GSK
*The lupus activity monitoring panel consists of six specialized biomarkers including: erythrocyte-bound C4d, anti-dsDNA by chemiluminescent immunoassay, complement C3 and C4, platelet-bound C4d, and the anti-C1q biomarker.
NR, not reportable.
*As defined by Garris et al. 2013, J Med Econ 16:5;667–77 during the pre-period.
**Measured using all available data prior to index date.
DCCI, Deyo-Charlson Comorbidity Index.
To cite this abstract in AMA style:
Concoff A, Moore-Schiltz L, Nadipelli V, O'Malley T, Worley K, Petrilla A, Zach D, Robinson S, Taiyari S, Jones B, Rubin B. The Real-World Utility of a Lupus Activity Monitoring Panel in the United States Community Rheumatologist Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-real-world-utility-of-a-lupus-activity-monitoring-panel-in-the-united-states-community-rheumatologist-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-real-world-utility-of-a-lupus-activity-monitoring-panel-in-the-united-states-community-rheumatologist-practice/