Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Acute injury to the anterior cruciate ligament (ACL) is a common cause of posttraumatic OA in humans, and ACL transection (ACLT) in rats is an established animal model of traumatic injury-induced OA. FX201, a helper-dependent adenovirus (HDAd)-based intra-articular (IA) gene therapy candidate designed to induce the production of an Interleukin (IL)-1 receptor antagonist (IL-1Ra) in the presence of inflammation, is in development as a potential therapeutic agent for OA. Here, we evaluated the effects of HDAd-ratIL-1Ra, the rat surrogate of FX201, when administered as a single IA injection in rats 1 week following ACLT surgery.
Methods: A total of 46 Sprague Dawley rats were assigned to 1 of 4 study groups: high-dose HDad-ratIL-1Ra (ACLT/HDAd-ratIL-1Ra; 2.4×108 viral particles [VP]/dose; n=12), low-dose HDAd-ratIL-1Ra (ACLT/HDAd-ratIL-1Ra; 3×107 VP/dose; n=12), vehicle (ACLT/vehicle; n=12), or sham/untreated (n=10). Rats underwent ACLT surgery in the right knee (except sham animals) under isoflurane anesthesia on Day -7. Seven days postsurgery (Day 1) rats received a single IA injection of HDAd-ratIL-1Ra or vehicle in the right knee joint under anesthesia. At Week 12, animals were sacrificed and whole right knee joints were harvested and analyzed for histopathology. For histopathological evaluation, whole right knee joints were stained with Safranin O fast green (SOFG) and hematoxylin and eosin and assessed using a semi-quantitative grading system (OARSI score) to score cartilage/bone and synovial membrane, respectively. Individual scores were recorded, and composite scores for each parameter were generated by the sum of all individual scores.
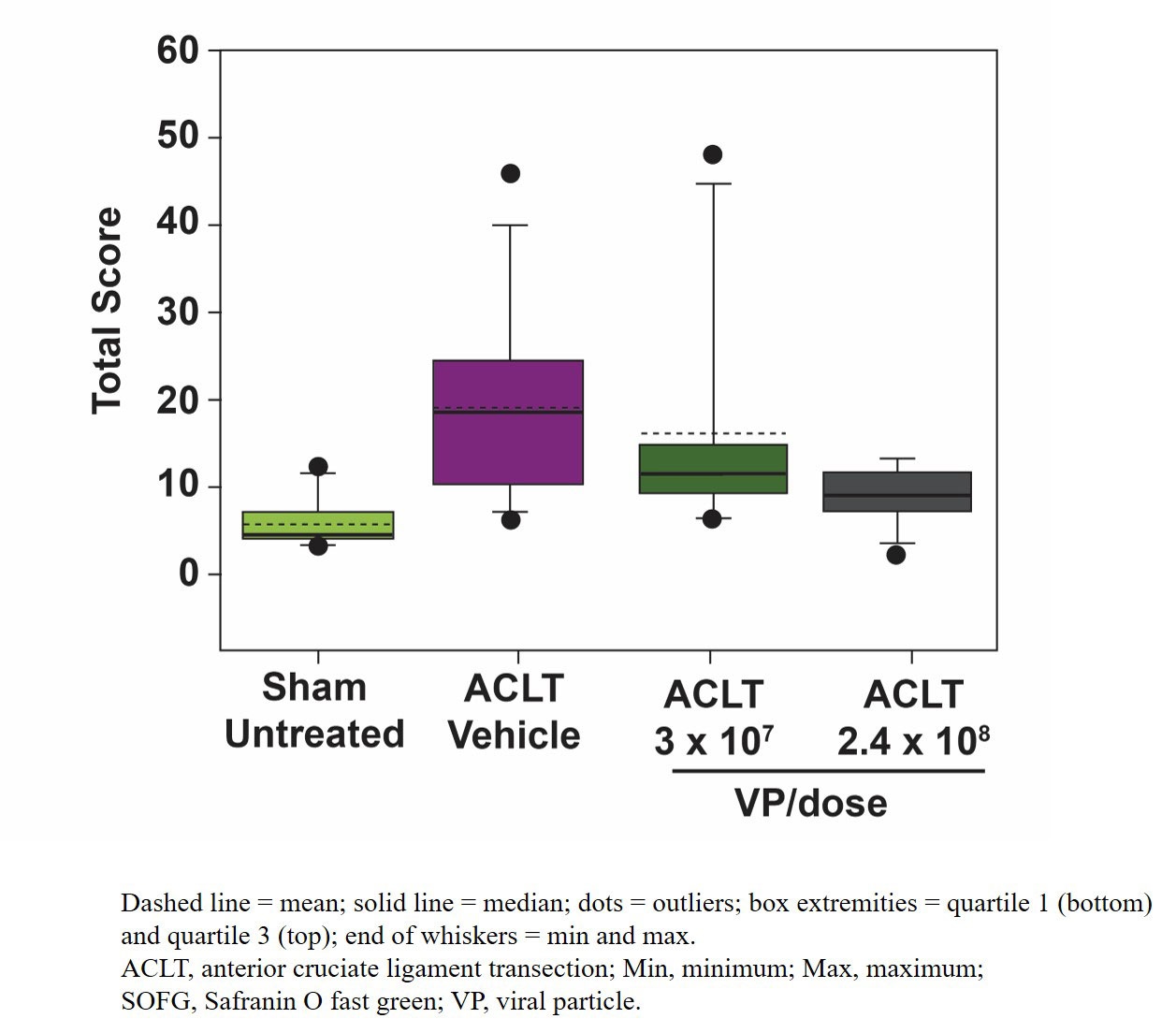
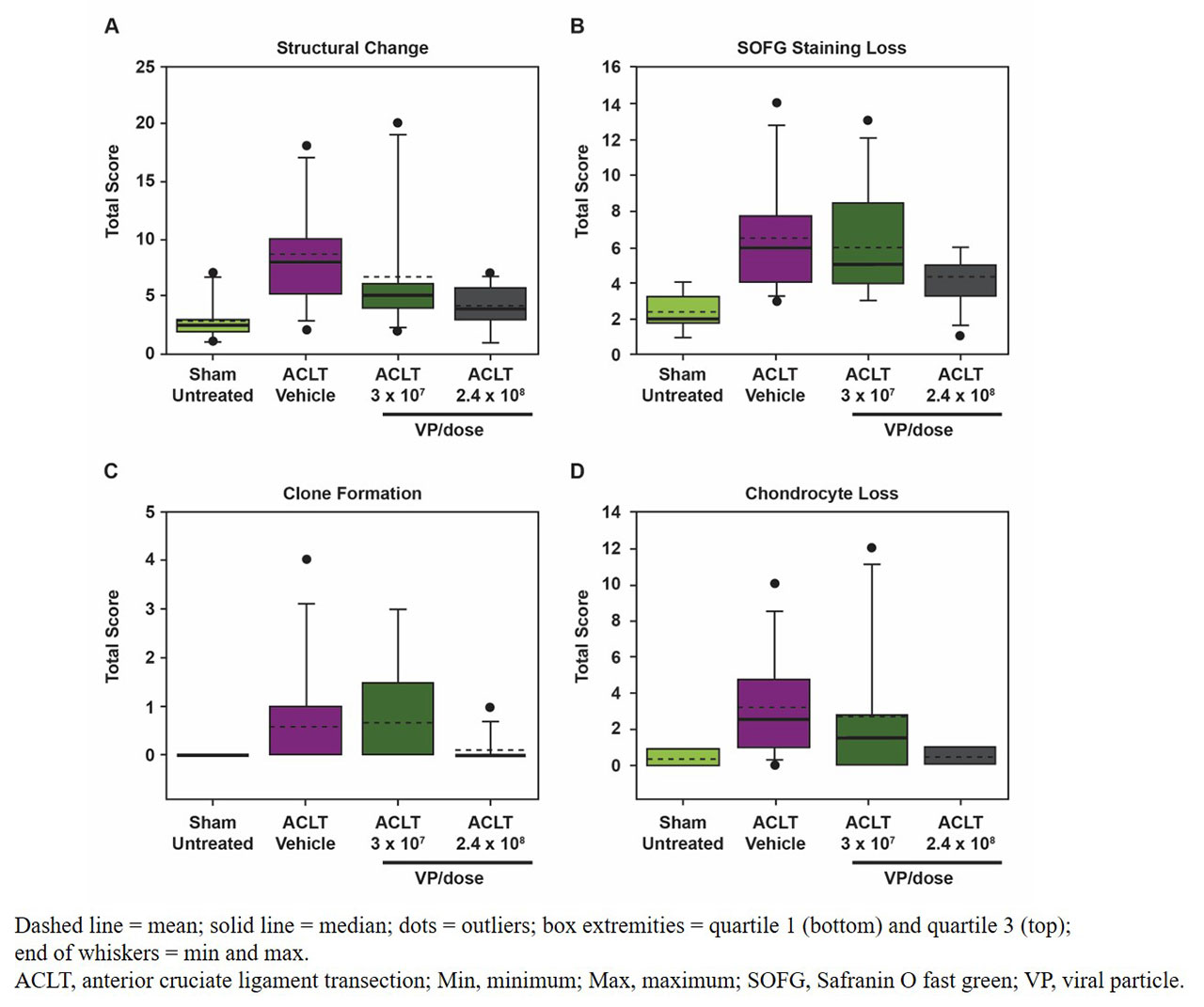
Results: In sham-operated rats, microscopic changes at Week 12 were limited to low incidence of superficial articular cartilage changes graded minimal in severity as assessed by surface irregularities with focal fibrillation/clefts/fissure, chondrocyte loss, and/or SOFG staining. All ACLT-operated rats developed OA microscopic changes that were minimal-to-severe in 1 or more of the examined articular compartments at Week 12. Among ACLT-operated rats, HDAd-ratIL-1Ra resulted in a dose-dependent decrease in composite scores for cartilage/bone compared with vehicle (Figure 1); these decreases are likely due to reduced severity of structural changes and chondrocyte loss with HDAd-ratIL-1Ra treatment compared with vehicle (Figure 2). Slight decreases in severity of SOFG staining loss and incidence of clone formation were also observed with HDAd-ratIL-1Ra treatment compared with vehicle (Figure 2). Furthermore, HDAd-ratIL-1Ra demonstrated dose-dependent decreases in composite scores of OA microscopic changes to the synovial membrane compared with vehicle (Figure 3); all synovial microscopic findings were minimal in severity.
Conclusion: A single administration of HDAd-ratIL-1Ra, the rat surrogate of FX201, resulted in a dose-dependent decrease in the incidence and severity of OA-related lesions to cartilage/bone and the synovial membrane 12 weeks postsurgery; these results support further development of FX201 as a potential therapeutic agent for OA.
To cite this abstract in AMA style:
Senter R, Boyce R, Chabicovksy M, Walsh Martin E, Langevin-Carpentier G, Bodick N. The Rat Homolog to FX201, a Gene Therapy in Development for the Treatment of Osteoarthritis, Demonstrates Dose-Dependent Decreases in the Severity of Cartilage and Bone Lesions Following Anterior Cruciate Ligament Transection [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-rat-homolog-to-fx201-a-gene-therapy-in-development-for-the-treatment-of-osteoarthritis-demonstrates-dose-dependent-decreases-in-the-severity-of-cartilage-and-bone-lesions-following-anterior-cruc/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-rat-homolog-to-fx201-a-gene-therapy-in-development-for-the-treatment-of-osteoarthritis-demonstrates-dose-dependent-decreases-in-the-severity-of-cartilage-and-bone-lesions-following-anterior-cruc/