Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: We have developed a new conceptual model that characterizes lupus into subtypes based on physician- and patient-reported measures: Type 1 is assessed by the rheumatologist and incorporates immunologic activity and organ involvement; Type 2 is based on patient report of fatigue, myalgia, mood disturbance, and perceived cognitive dysfunction. We have previously defined Type 2 symptoms based on the ACR’s Fibromyalgia Severity Score (FSS), and here we seek to assess the impact of Type 1 and 2 SLE activity on the NIH’s Patient-Reported Outcomes Measurement Information System (PROMIS) measures.
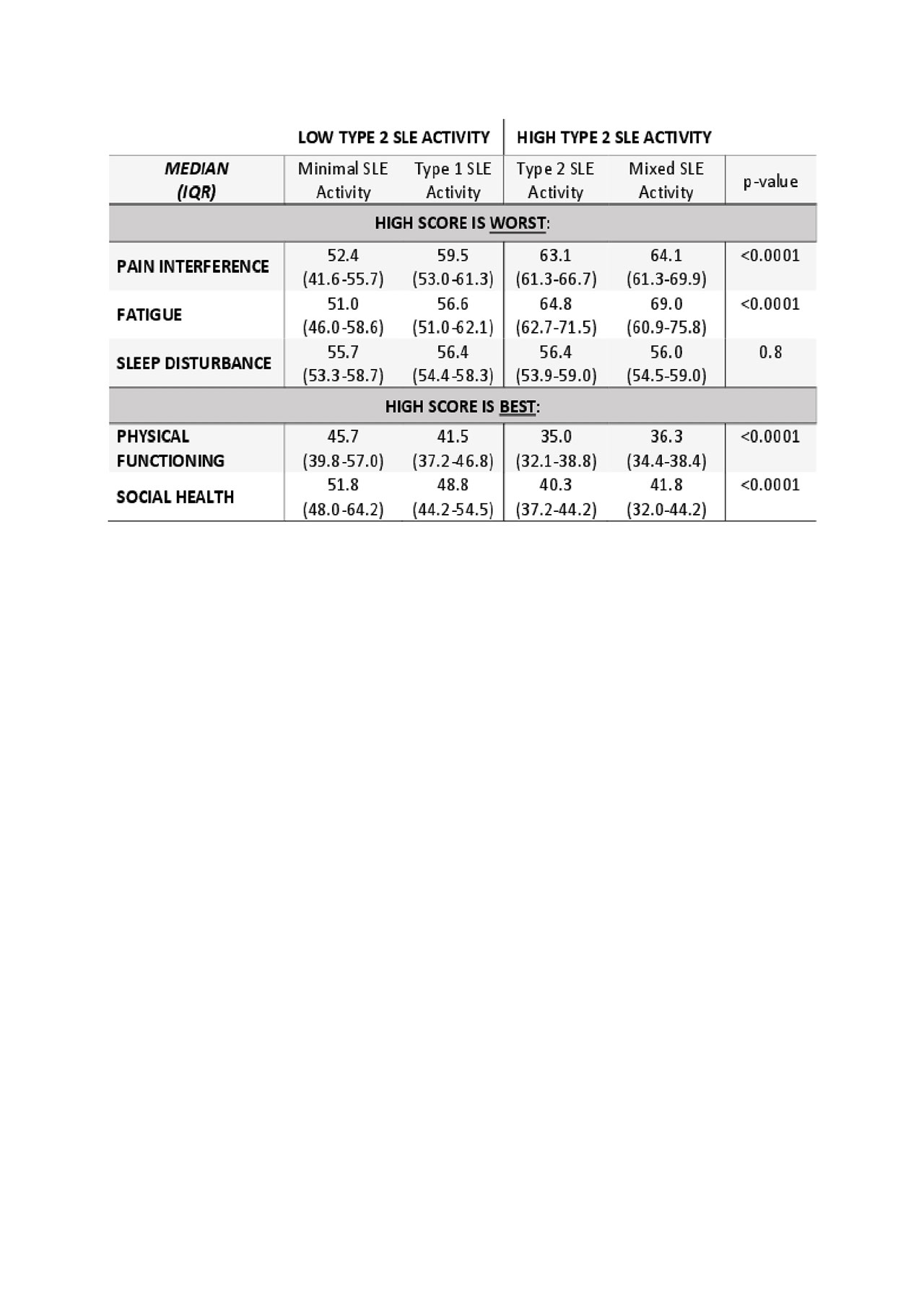
Methods: Data on physician- and patient-reported measures of disease activity were collected on women with SLE enrolled in a prospective registry at a single academic center from 2018-2019. Patients were classified as having Type 1 SLE activity (SLEDAI ≥6, clinical SLEDAI ≥4, or active nephritis), Type 2 SLE activity (FSS >10), Mixed (both Type 1 and Type 2 SLE activity), or minimal (neither Type 1 or Type 2 SLE activity). We compared the PROMIS composite measures of pain interference, physical functioning, sleep disturbance, fatigue, and ability to participate in social roles and activities between groups using the Wilcoxon rank-sum test. A PROMIS score of 50 is the national average, and a score difference of 5 (half standard deviation) is clinically significant.
Results: Data were collected from 116 women with SLE of whom 63% were African American with an average age 43. Compared to the US general population, patients with either Mixed or Type 2 SLE activity have moderately worse scores (1-2 standard deviation difference), while patients with Type 1 SLE activity alone have only mildly worse scores (< 1 standard deviation difference) in physical functioning, ability to participate in social roles and activities, fatigue, and pain interference. Patients with Minimal SLE activity were the least limited by pain, suffered from less interference with physical and social functioning, and their scores were not clinically significantly different (within a half standard deviation) from the general population. Despite the large differences in fatigue, all SLE groups reported similar degrees of mild sleep disturbance (Table 1).
Conclusion: The presence or absence of Type 2 symptoms was the primary determinant of PROMIS scores in pain, physical functioning, fatigue, and social well-being. This analysis demonstrates that multiple domains of the PROMIS-29 correspond to the Type 1 and 2 SLE model and may be useful in measuring levels of Type 1 and 2 SLE activity. Next steps include identifying and validating the optimal PROMIS domains that can be used to determine current Type 2 SLE activity.
To cite this abstract in AMA style:
Harris N, Eudy A, Clowse M, Criscione-Schreiber L, Doss J, Sadun R, Rogers J, Sun K. The PROMIS-29 as a Measure of Type 1 and 2 SLE Activity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-promis-29-as-a-measure-of-type-1-and-2-sle-activity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-promis-29-as-a-measure-of-type-1-and-2-sle-activity/