Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The 2023 ACR/EULAR Antiphospholipid Syndrome (APS) Classification Criteria (CC) focus on IgG and IgM anticardiolipin (aCL) and anti-β2-glycoprotein-I antibodies (aβ2GPI). IgA isotypes were excluded due to limited evidence linking isolated IgA positivity to thrombosis, uncertain incremental value beyond established criteria, and a lack of assay standardization. However, emerging data from the general population suggest that individuals with IgA aβ2GPI may face an elevated risk of atherosclerotic cardiovascular disease, potentially due to impaired cholesterol efflux capacity and endothelial injury. Our primary objective was to compare the demographic, clinical, and laboratory characteristics of aCL and/or aβ2GPI IgA-positive versus IgA-negative patients. A secondary objective was to identify clinical and serological factors independently associated with IgA positivity. TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack This page is in Dutch Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate Dutch to Turkish Never translate Dutch Never translate www.abstractscorecard.com
Methods: The APS ACTION registry uses a web-based system to capture patient demographics, APS-related history, and medications. Inclusion requires aPL positivity per Revised Sapporo APS CC, confirmed within one year prior to enrollment. Patients are followed every 12±3 months with clinical assessments and blood collection. For this cross-sectional analysis of baseline data, we first compared demographic, clinical, and laboratory features between IgA-positive (aCL and/or aβ2GPI IgA >20 U; analysis repeated with >40 U cutoff) and IgA-negative (< 20 U) patients using chi-square or Fisher’s exact tests, based on APS ACTION Core Lab results (BioFlash, Werfen). We then conducted multivariate logistic regression to identify factors independently associated with IgA positivity.TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack
Results: Of 1,252 patients recruited as of January 2025, 706 (56%) had baseline aCL/aβ2GPI IgA results. Using the >20U threshold recommended by the manufacturer, the IgA-positive group (n: 238 [34%]) had significantly higher rates of venous events, thrombocytopenia, heart valve disease, and triple/double aPL-positivity. Diabetes, hyperlipidemia, and single aPL-positivity were significantly higher in IgA-negative group (Table 1). In the multivariate analysis, after adjusting for the aPL profile, history of venous events, heart valve disease, thrombocytopenia, systemic lupus erythematosus (SLE), and any traditional cardiovascular disease risk factor, IgA-positivity was significantly associated with triple/double aPL-positivity, SLE, and thrombocytopenia (Table 2). When the IgA positivity cut-off of > 40U used: a) there was no change in the univariate results, except that diabetes, and hyperlipidemia no longer differed between the groups; and b) in multivariate analysis, IgA-positivity was significantly associated with triple/double aPL-positivity and history of venous events (data not shown). This page is in English Translate to Turkish AfrikaansAlbanianAmharicArabicArmenianAzerbaijaniBengaliBulgarianCatalanCroatianCzechDanishDutchEnglishEstonianFinnishFrenchGermanGreekGujaratiHaitian CreoleHebrewHindiHungarianIcelandicIndonesianItalianJapaneseKannadaKazakhKhmerKoreanKurdish (Kurmanji)LaoLatvianLithuanianMalagasyMalayMalayalamMalteseMaoriMarathiMyanmar (Burmese)NepaliNorwegianPashtoPersianPolishPortuguesePunjabiRomanianRussianSamoanSimplified ChineseSlovakSlovenianSpanishSwedishTamilTeluguThaiTraditional ChineseTurkishUkrainianUrduVietnameseWelsh Always translate English to Turkish Never translate English Never translate www.abstractscorecard.com
Conclusion: In our well-established international cohort of persistently aPL-positive (lupus anticoagulant, aCL IgG/M, and/or aβ2GPI IgG/M) patients, concomitant aCL/aβ2GPI IgA-positivity correlates with a more severe clinical and, especially, serological phenotype. Future prospective analysis of the registry will help us further clarify the clinical significance of aCL/aβ2GPI IgA antibodies in aPL-positive patients.TRANSLATE with xEnglishArabicHebrewPolishBulgarianHindiPortugueseCatalanHmong DawRomanianChinese SimplifiedHungarianRussianChinese TraditionalIndonesianSlovakCzechItalianSlovenianDanishJapaneseSpanishDutchKlingonSwedishEnglishKoreanThaiEstonianLatvianTurkishFinnishLithuanianUkrainianFrenchMalayUrduGermanMalteseVietnameseGreekNorwegianWelshHaitian CreolePersian TRANSLATE with COPY THE URL BELOW BackEMBED THE SNIPPET BELOW IN YOUR SITE Enable collaborative features and customize widget: Bing Webmaster PortalBack
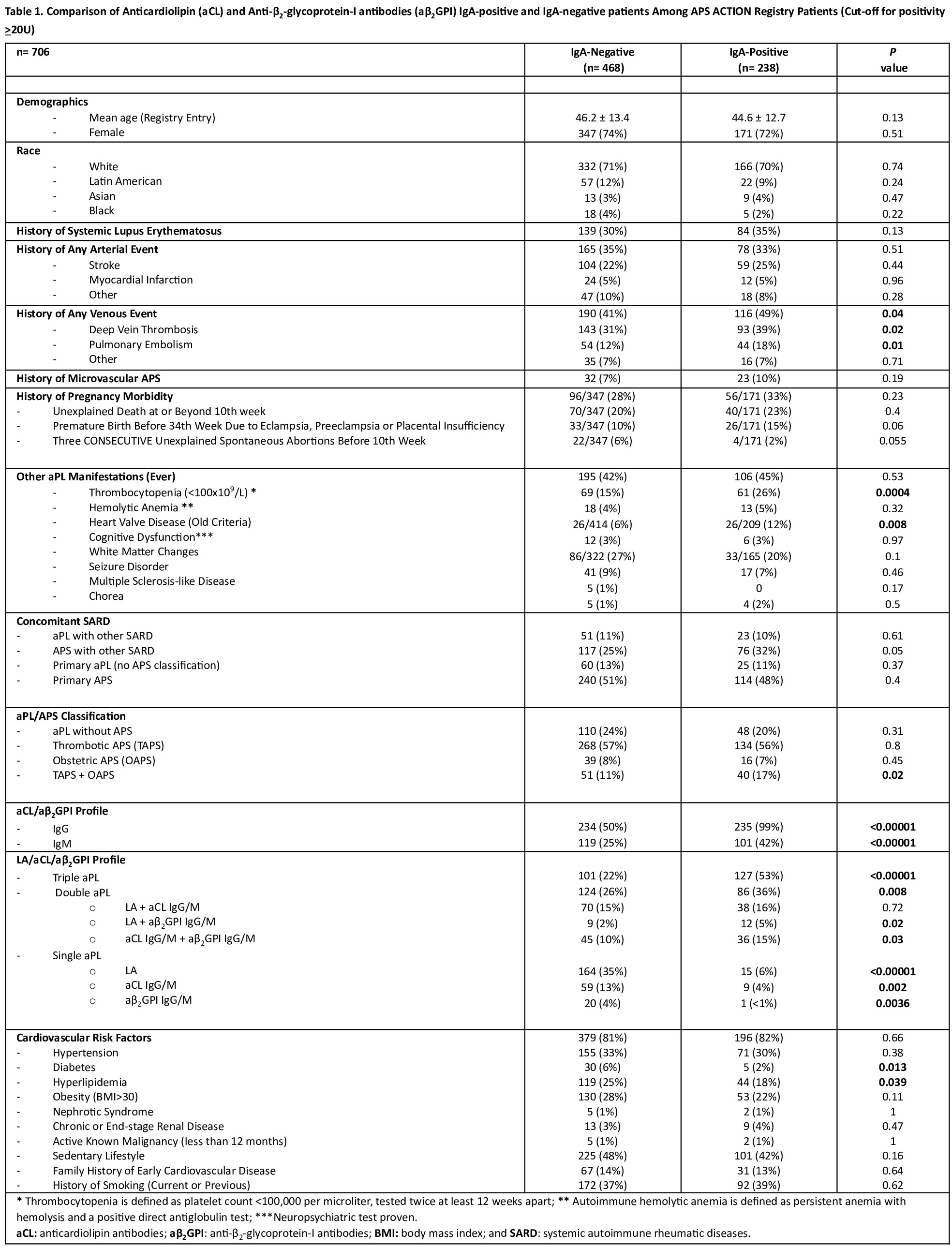 Table 1. Comparison of Anticardiolipin (aCL) and Anti-β2-glycoprotein-I antibodies (aβ2GPI) IgA-positive and IgA-negative patients Among APS ACTION Registry Patients (Cut-off for positivity >20U)
Table 1. Comparison of Anticardiolipin (aCL) and Anti-β2-glycoprotein-I antibodies (aβ2GPI) IgA-positive and IgA-negative patients Among APS ACTION Registry Patients (Cut-off for positivity >20U)
.jpg) Table 2: Predictors of Anticardiolipin (aCL) and Anti-β2-glycoprotein-I antibodies (aβ2GPI) IgA Positivity Among APS ACTION Registry Patients (Cut-off for positivity >20U) (multivariate logistic regression)
Table 2: Predictors of Anticardiolipin (aCL) and Anti-β2-glycoprotein-I antibodies (aβ2GPI) IgA Positivity Among APS ACTION Registry Patients (Cut-off for positivity >20U) (multivariate logistic regression)
To cite this abstract in AMA style:
Sahin E, Zuo Y, Andrade D, Tektonidou M, Pengo V, Radin M, López pedrera C, Paredes-Ruiz D, Belmont H, Fortin P, WAHL D, Branch W, Gerosa M, Ramires de Jesus G, Atsumi T, Efthymiou M, Tincani A, Rodriguez-Almaraz E, Petri M, Cervera R, Willis R, Devreese K, Bertolaccini M, Cohen H, Knight J, Erkan D. The Prevalence and Clinical Significance of IgA Anticardiolipin and Anti-β2-Glycoprotein-I Antibody Isotypes in Antiphospholipid Antibody Positive Patients: Descriptive Results from the Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-prevalence-and-clinical-significance-of-iga-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-antibody-isotypes-in-antiphospholipid-antibody-positive-patients-descriptive-results-from-the-antiphosp/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-prevalence-and-clinical-significance-of-iga-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-antibody-isotypes-in-antiphospholipid-antibody-positive-patients-descriptive-results-from-the-antiphosp/
