Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: A simple, scalable tool that identifies psoriasis patients at high risk for developing PsA could improve early detection and facilitate early intervention. Our overall objective is to develop an accurate risk prediction model for the development of PsA in patients with psoriasis. This analysis focused on clinical predictors.
Methods: In this longitudinal cohort study we analyzed data from the International Psoriasis and Arthritis Team (IPART) study, a prospective cohort of psoriasis patients without PsA at the time of enrollment. The participants have been followed prospectively and their PsA status has been assessed annually by a rheumatologist. Information about their demographics, psoriasis characteristics, co-morbidities and musculoskeletal symptoms, was used to develop prediction models for PsA. Serial logistic regression models were fitted with a defined set of time-varying covariates in order to identify the optimal combination of covariates to construct risk prediction models. Prediction models were set to estimate the risk of developing PsA over 1 and 5 years respectively. Each model was adjusted for psoriasis duration. Model selection was based on backward elimination approach (p< 0.10). Receiver operating characteristics curves were plotted and the predictive performance was summarized by the area under the curve (AUC).
Results: A total of 635 psoriasis patients, followed from 2016 to 2020, were analyzed (mean duration of follow up 7.7 years). 75 patients developed PsA during the study period. Univariate analysis identified various candidate predictors of PsA including the severity of musculoskeletal symptoms, psoriatic nail lesions, iritis, psoriasis type, location and severity and the use of non-biologic systemic medications or phototherapy (Table 1). The risk of developing PsA within 1 year was predicted by younger age, patient global health and pain severity (AUC 76.8, 95% confidence interval (CI) 68.5, 85.1, Figure 2). The risk of developing PsA within 5 years was predicted by psoriatic nail lesion, health assessment questionnaire disability index (HAQ-DI), Functional Assessment of Chronic Illness Therapy (FACIT) fatigue scale and use of systemic non-biologic medication or phototherapy (AUC 71.9, 95% CI 65.2, 78.6, Figure 3).
Conclusion: The development of PsA within clinically meaningful time frames can be predicted with reasonable accuracy for psoriasis patients. Additional work is underway to optimize the current models and validate them in external cohorts of psoriasis patients.
 Table 1 – Candidate predictors of PsA in Univariate Models at 1 year and 5 years (p < 0.10)
Table 1 – Candidate predictors of PsA in Univariate Models at 1 year and 5 years (p < 0.10)
 Figure 1 – The Risk of Developing PsA within 1 year – Cox Proportional Hazards Model
Figure 1 – The Risk of Developing PsA within 1 year – Cox Proportional Hazards Model
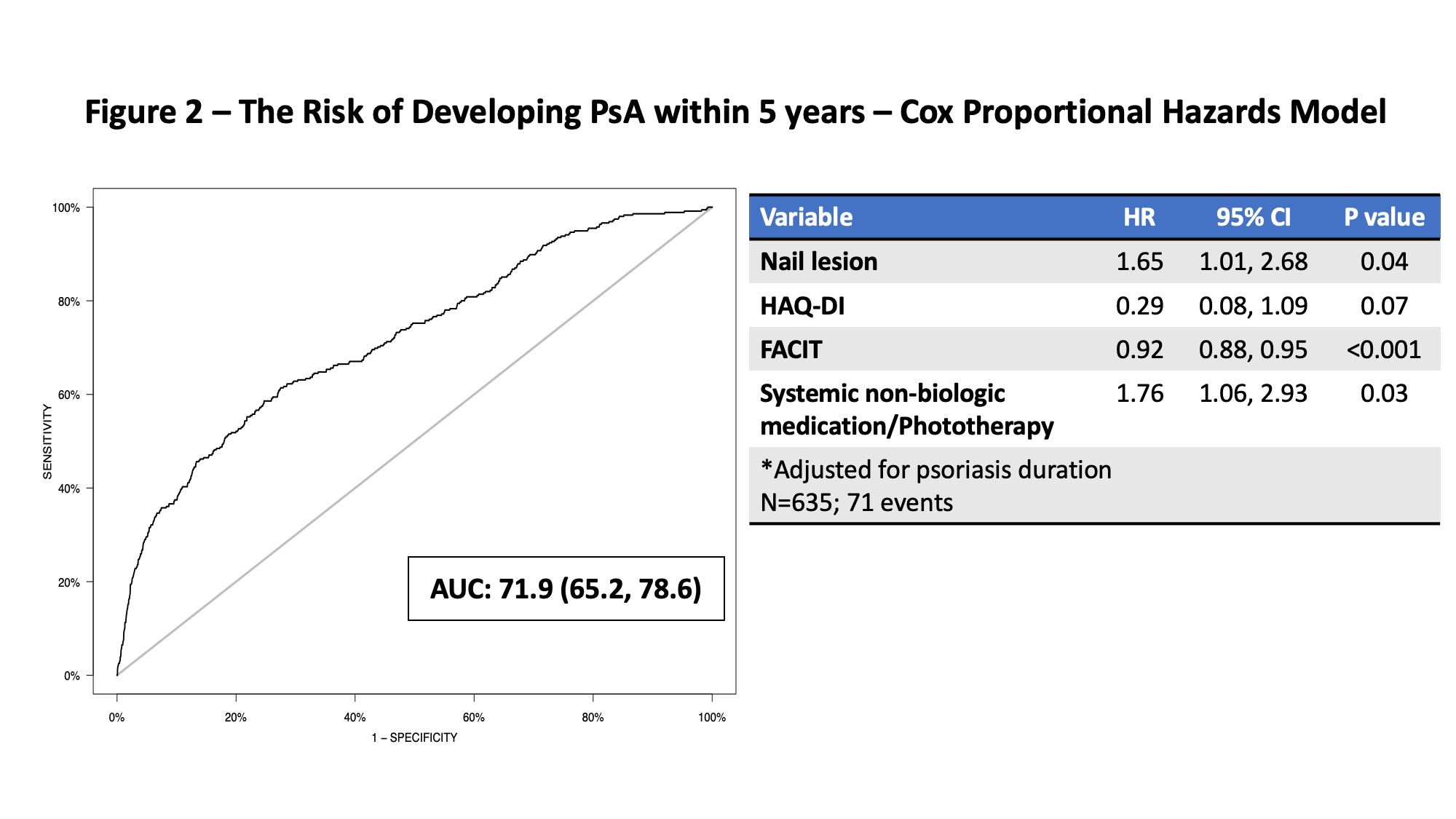 Figure 2 – The Risk of Developing PsA within 5 years – Cox Proportional Hazards Model
Figure 2 – The Risk of Developing PsA within 5 years – Cox Proportional Hazards Model
To cite this abstract in AMA style:
Eder L, Lee K, Chandran V, Widdifield J, Drucker A, Ritchlin C, Rosen C, Cook R, Gladman D. The Prediction of Psoriatic Arthritis Tool (PRESTO) Study – Interim Report [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-prediction-of-psoriatic-arthritis-tool-presto-study-interim-report/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-prediction-of-psoriatic-arthritis-tool-presto-study-interim-report/
