Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Composite measures of disease activity are used in psoriatic arthritis (PsA), but their relative performance and contributions of individual components to overall scores are unclear. Primary results were reported* from a phase 3 trial that randomized methotrexate (MTX)- and biologic-naïve patients with active PsA to weekly: MTX 20 mg; etanercept (ETN) 50 mg; or ETN 50 mg + MTX 20 mg. At week 24, the ETN-containing arms were significantly more effective than MTX monotherapy in achieving an American College of Rheumatology 20 response (primary endpoint) and Minimal Disease Activity response (key secondary endpoint). Compared with MTX monotherapy, the ETN-containing arms also had a composite score change from baseline at week 24 that was larger with Psoriatic Arthritis Disease Activity Score (PASDAS) and numerically higher with Activity Index for Psoriatic Arthritis (DAPSA).* Here we further examine the trial composite measures by analyzing contributions of individual components to the change in overall composite scores from baseline at week 24 for: PASDAS, DAPSA, Disease Activity Score (DAS28-CRP), Clinical Disease Activity Index (CDAI), and Simplified Disease Activity Index (SDAI).
Methods: Using the full analysis set of 851 patients, the median and mean (95% CI) contribution of each individual domain change to the composite measure change from baseline at week 24 was calculated. Analyses were repeated for PASDAS in patient subsets with enthesitis or dactylitis at baseline.
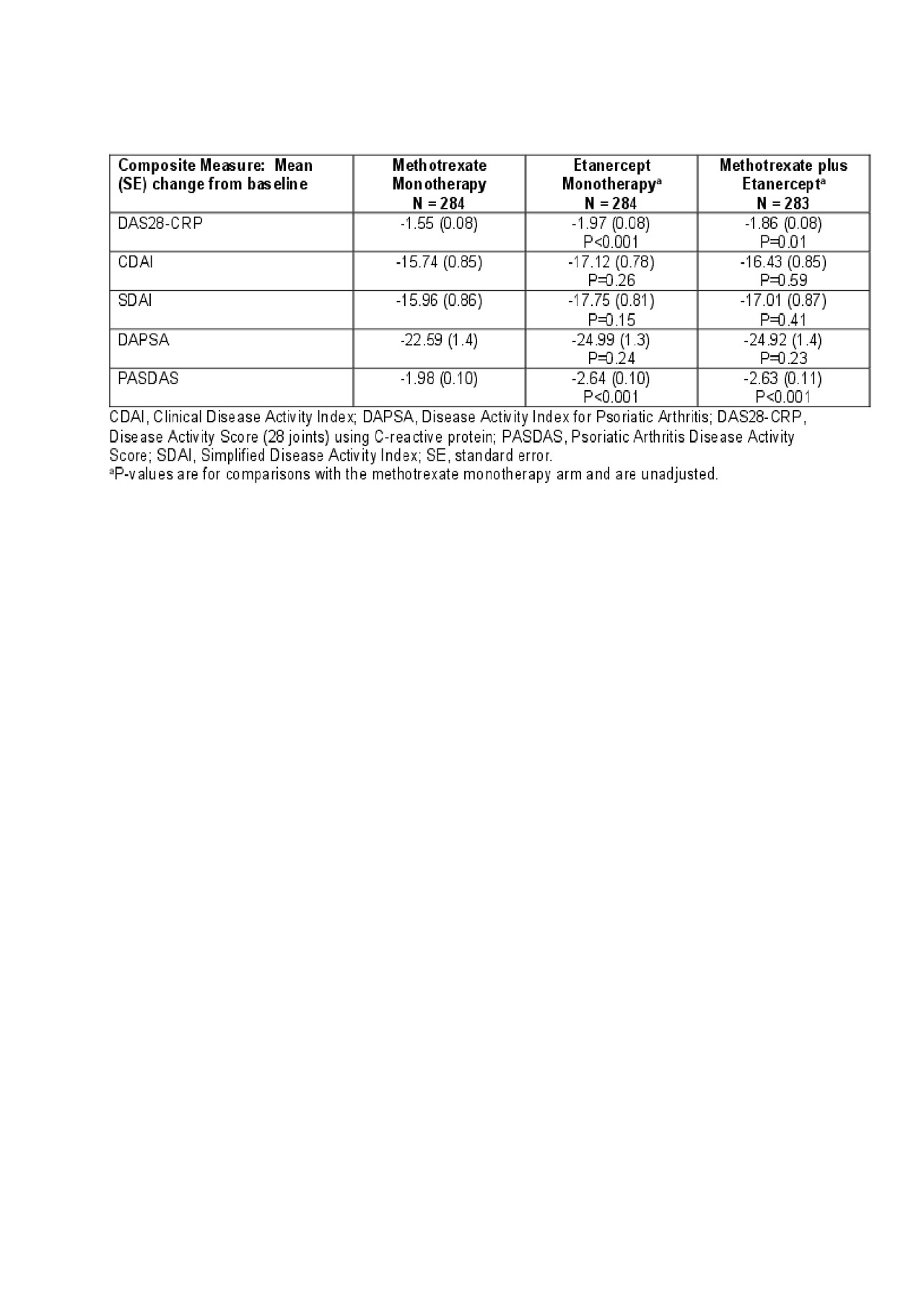
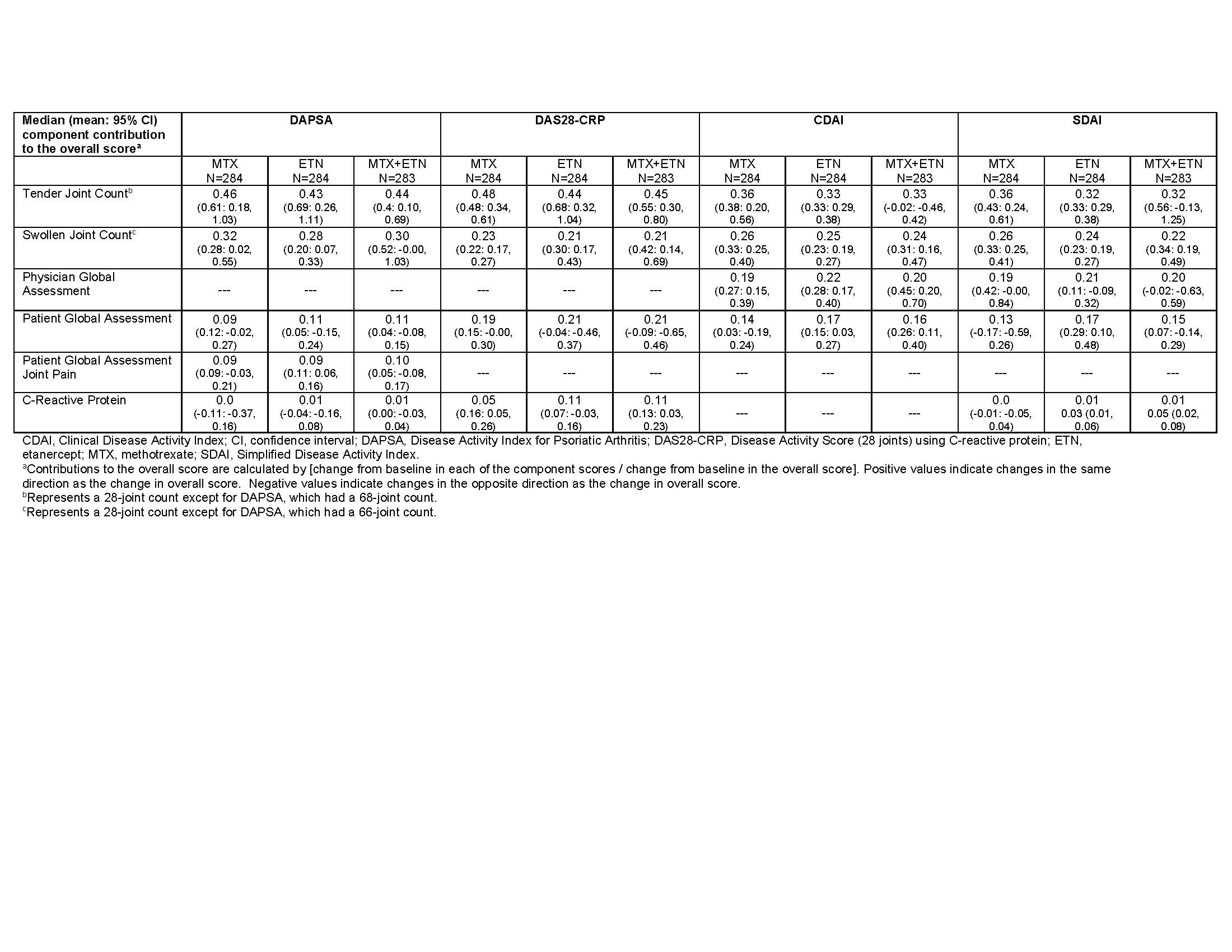
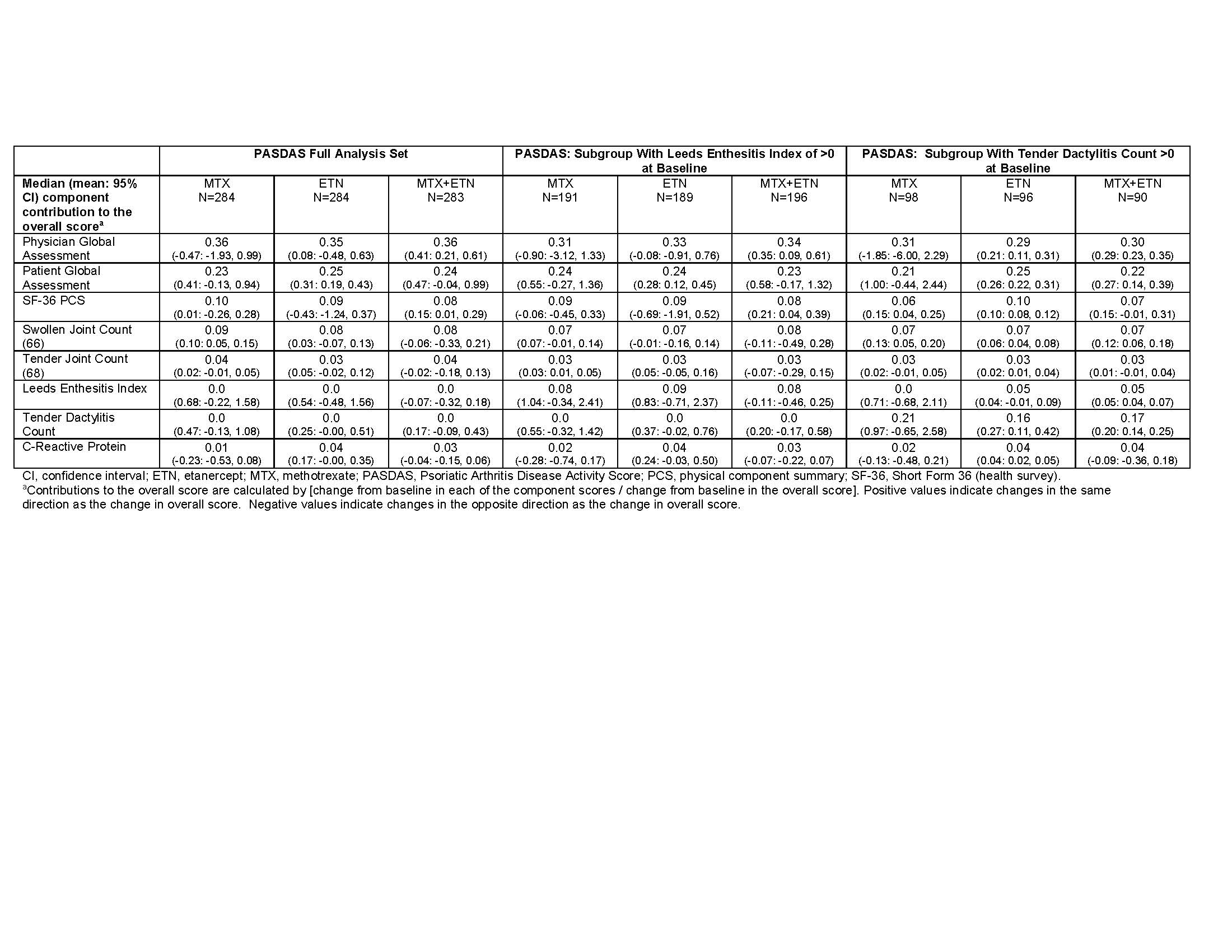
Results: The ETN-containing arms had a greater composite score change from baseline at week 24 compared with MTX monotherapy; PASDAS and DAS28-CRP had relatively greater changes compared with DAPSA, CDAI, and SDAI (Table 1). For DAPSA, DAS28-CRP, CDAI, and SDAI, the joint count changes contributed most to score changes, while the global assessment changes contributed less (Table 2). In contrast, the global assessment changes contributed the most to the PASDAS score changes, with less contribution from joint count changes (Table 3). Contribution of enthesitis change to the PASDAS score change in those with enthesitis at baseline was similar to that of the joint counts; in those with dactylitis at baseline, the contribution of dactylitis change was more than that of the joint counts. Except for DAS28-CRP, the composite score changes showed minimal contributions from changes in CRP. Overall, the contributions of changes in the individual components to the composite score changes were similar between treatment arms.
Conclusion: Results show that changes in “joint-focused” composite endpoints (DAPSA, DAS28-CRP, CDAI, and SDAI) are driven most by joint count changes and less by global assessment changes. Change in PASDAS, which captures a wider range of disease domains, was driven most by global assessment changes (and somewhat by physical function) and less by other domain changes depending on the patient population. Changes in CRP consistently contributed less than other components. Results were consistent across treatment arms.
*Mease et al. Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851 [Epub ahead of print].
Linda Rice at Amgen Inc assisted in writing this abstract.
To cite this abstract in AMA style:
Coates L, Merola J, Mease P, Ogdie A, Gladman D, Strand V, van Mens L, Liu L, Yen P, Collier D, Kricorian G, Chung J, Helliwell P. The Performance Characteristics of Composite Measures Used in a Randomized Trial Examining Etanercept and Methotrexate as Monotherapy or in Combination in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-performance-characteristics-of-composite-measures-used-in-a-randomized-trial-examining-etanercept-and-methotrexate-as-monotherapy-or-in-combination-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-performance-characteristics-of-composite-measures-used-in-a-randomized-trial-examining-etanercept-and-methotrexate-as-monotherapy-or-in-combination-in-patients-with-psoriatic-arthritis/