Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: It has been over 20 years since the OMERACT core outcome set (COS) to measure in clinical trials with people who have hip and/or knee osteoarthritis (OA) was presented. An OMERACT-OARSI Working Group was established to update this COS using contemporary OMERACT methodologies.
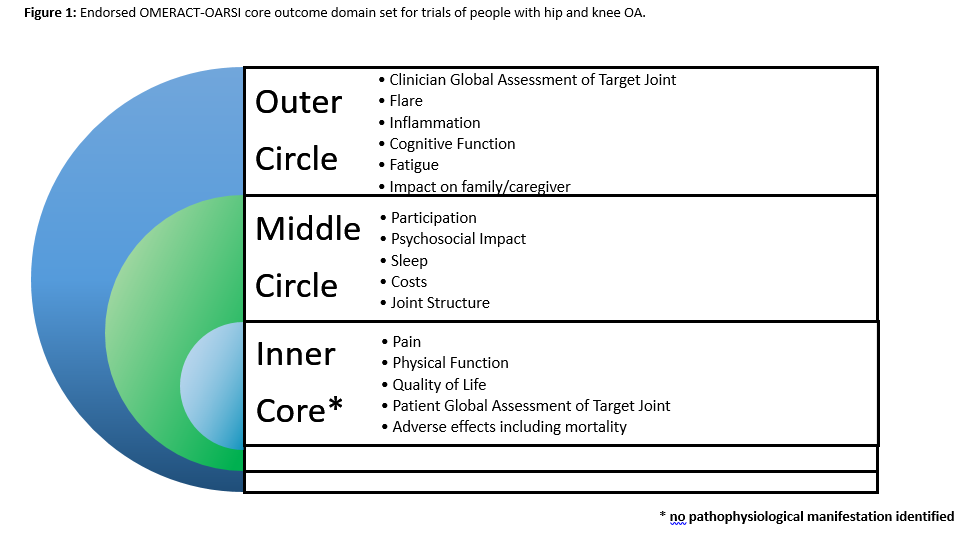
Methods: A review of the COMET database of COS was undertaken to identify all domains reported in previous COS that including individuals with hip and/or knee OA. These were presented in a series of patient and public (PP) meetings involving 70 individuals across the UK, Australia and Canada to review the domain list and identify additional important domains. Based on these, a three-round international Delphi survey was undertaken recruiting patients, healthcare professionals, researchers and industry representatives to gain consensus on key domains which should be included in this core domain set. Using the Delphi results, an OMERACT ‘onion’ was formulated with the following rules: Inner core inclusion (mandatory for all trials) was defined as domains reported as ‘critical’ to assess by over 70% of Delphi responses in the patient AND others stakeholder groups; Middle circle inclusion (recommended by optional in trials) was defined as domains reported as ‘critical’ to assess by over 70% of Delphi responses in the patient OR others stakeholder groups; Outer circle inclusion was defined as areas which need further research with insufficient evidence supporting middle circle placement. The findings were discussed at OMERACT2018 and a consensus vote was obtained on the core domain set.
Results: From the COMET review, four previous COS were identified including the 1997 OMERACT core outcome set. These were reviewed across the PP meetings to identify 50 potential domains which formed the Delphi survey. In total 424 (217 patients; 207 non-patients) contributed data to the Delphi exercise from 25 different countries. The OMERACT2018 delegates (129 participants) voted on those domains which met the criteria for inclusion. From this, four domains gained agreement on inner core inclusion to be mandatory in all clinical trials with people with hip and/or knee OA: ‘pain’ (100% of vote), ‘physical function’ (100%), ‘quality of life’ (90%) and ‘patient global assessment of the target joint’ (91%) in addition to the mandated core domain of ‘adverse events including mortality’. Adherence was specified as a critical contextual factor which should be assessed. The core domain set (inner core, middle and outer circle) is presented in Figure 1.
Conclusion: The updated core domain set for hip and/or knee OA has been agreed. We will now build on this work to determine which instruments should be recommended to measure each of the inner core domains based on the OMERACT Filter 2.1 guidance.
To cite this abstract in AMA style:
Smith TO, Hawker G, Hunter DJ, March L, Shea B, Christensen R, Guillemin F, Terwee C, Williamson P, Roos EM, Loeser R, Schnitzer TJ, Kloppenburg M, Neogi T, Ladel C, Kaiser U, Mobasheri A, Arden NK, Hochberg MC, Tennant A, de Wit M, Tugwell P, Conaghan PG. The Omeract-Oarsi Core Set of Outcome Domains to Measure in Clinical Trials for People with Hip and/or Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-omeract-oarsi-core-set-of-outcome-domains-to-measure-in-clinical-trials-for-people-with-hip-and-or-knee-osteoarthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-omeract-oarsi-core-set-of-outcome-domains-to-measure-in-clinical-trials-for-people-with-hip-and-or-knee-osteoarthritis/