Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
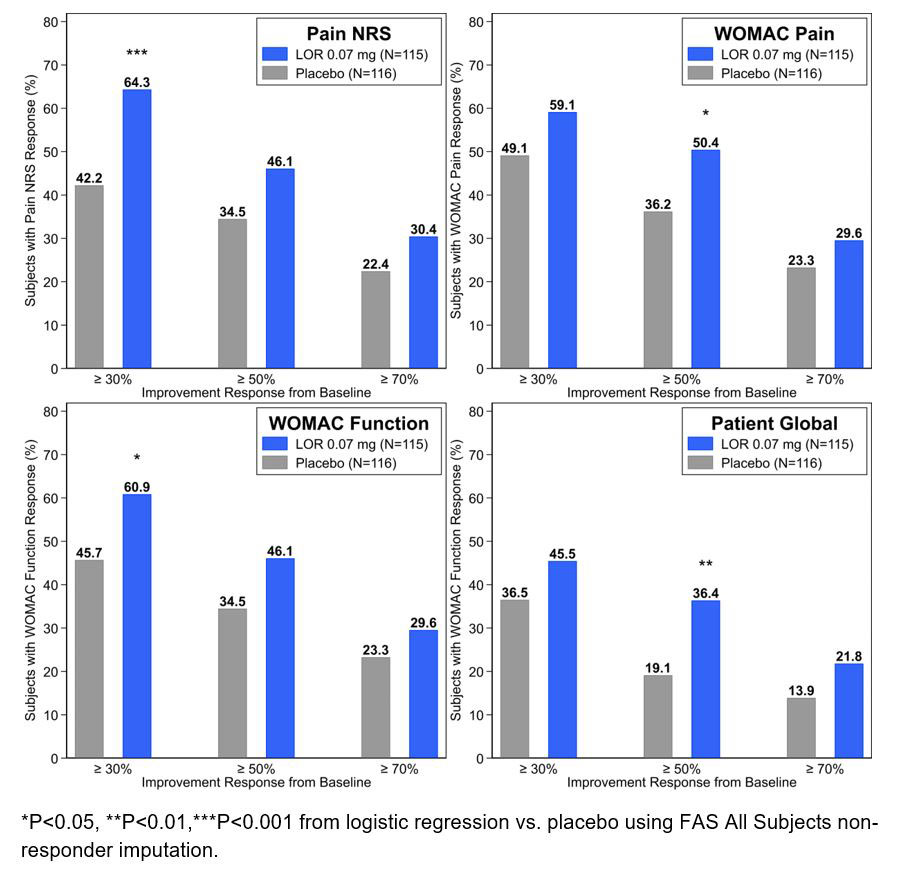
Background/Purpose: Lorecivivint (LOR, SM04690) is a small-molecule, intra-articular (IA) CLK/DYRK1A inhibitor which modulates the Wnt pathway and has demonstrated beneficial effects on patient-reported outcomes (PROs) in two Phase 2 trials in subjects with knee OA relative to placebo (PBO). Representing PROs as discrete threshold responses instead of as changes in mean point estimates may better evaluate clinically meaningful benefits experienced by trial subjects. This post hoc analysis was conducted to measure the proportion of subjects treated with LOR in a 24-week Phase 2b study who achieved 30%, 50%, or 70% improvement over baseline by Pain Numeric Rating Scale (NRS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, WOMAC Function, and Patient Global Assessment (PtGA). Results from the Phase 3 selected dose of 0.07 mg LOR are presented here.
Methods: Subjects had ACR-defined knee OA, Kellgren-Lawrence (KL) grades 2-3, and Pain NRS scores ≥4 and ≤8 in the target knee and < 4 in the contralateral knee. A single, 2mL, IA injection of 0.03 mg, 0.07 mg, 0.15 mg, or 0.23 mg LOR or vehicle PBO was given in the target knee at baseline. The proportion of subjects meeting thresholds of 30%, 50%, and 70% improvements over baseline in the PROs of weekly average of daily Pain NRS [0-10], WOMAC Pain [0-100], WOMAC Function [0-100], and PtGA [0-100] at Week 12 was determined. The odds ratios (OR) of achieving each threshold improvement level were calculated.
Results: 635 subjects (91.4%) completed the study (mean age 59.0±8.5 years, BMI 29.0±4.0 kg/m2, female 58.4%, KL3 57.3%). Treatment with 0.07 mg LOR versus PBO at Week 12 led to 1) significantly (P< 0.05) increased odds of having a 30% response in Pain NRS (OR 2.47 [1.45, 4.19]) and WOMAC Function (OR 1.86 [1.10, 3.12]), 2) significantly increased odds of achieving a 50% response for WOMAC Pain (OR 1.79 [1.06, 3.03]) and PtGA (OR 2.28 [1.25, 4.16]), and 3) numerically, but not significantly, more subjects achieved a 70% response in all PROs. Improvements were maintained through Week 24.
Conclusion: LOR, in development as a potential disease-modifying knee OA drug, demonstrated significantly higher odds ratios of achieving and maintaining clinically relevant improvements in PROs compared to placebo from Week 12 through Week 24. Phase 3 studies are ongoing.
To cite this abstract in AMA style:
Yazici Y, Kennedy S, Swearingen C, Tambiah J. The Novel, Intra-articular CLK/DYRK1A Inhibitor Lorecivivint (LOR; SM04690), Which Modulates the Wnt Pathway, Improved Responder Outcomes in Subjects with Knee Osteoarthritis: A Post Hoc Analysis from a Phase 2b Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-novel-intra-articular-clk-dyrk1a-inhibitor-lorecivivint-lor-sm04690-which-modulates-the-wnt-pathway-improved-responder-outcomes-in-subjects-with-knee-osteoarthritis-a-post-hoc-analysis-from/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-novel-intra-articular-clk-dyrk1a-inhibitor-lorecivivint-lor-sm04690-which-modulates-the-wnt-pathway-improved-responder-outcomes-in-subjects-with-knee-osteoarthritis-a-post-hoc-analysis-from/