Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Axia is a novel digital therapeutic (DTx) specifically designed for patients with axial spondyloarthritis (axSpA), in compliance with the European Medical Device Regulation (MDR). Axia delivers an innovative, app-based intervention combining individualized home exercise programs, patient education, and comprehensive disease management, incorporating structured gamification elements to support long-term adherence. To date, no DTx targeting rheumatic diseases has been convincingly demonstrated to improve disease activity. The Bechterew-App Trial was designed as a nationwide, randomized, controlled interventional trial (RCT) to assess the efficacy of Axia over a 12-week period in German axSpA patients with stable pharmacotherapy.
Methods: In this 12-week RCT, 200 patients with axSpA receiving stable pharmacotherapy were randomized (1:1) to either using Axia (intervention group) or treatment as usual (control group) (Figure 1 and Figure 2). The primary endpoints are improvements in disease activity, measured by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI); disease-specific functionality, assessed by the Bath Ankylosing Spondylitis Functional Index (BASFI); and disease-specific quality of life, evaluated using the Ankylosing Spondylitis Quality of Life questionnaire (ASQoL). Secondary endpoints include responder analyses based on the Assessment of Spondyloarthritis International Society (ASAS)20 and ASAS40 response criteria.
Results: Of the 200 axSpA patients enrolled in the trial, 186 participants (95 in the intervention group and 91 in the control group) completed the study (Figure 2). Apart from a slightly higher proportion of female participants, no significant differences were observed between the groups at baseline (Figure 1). Participants in the Axia intervention group demonstrated significant and clinically meaningful improvements in disease activity (BASDAI: –1.66 [SD 1.41] vs. control: –0.11 [1.15]; p < 0.001), functional status (BASFI: –1.12 [1.40] vs. control: 0.06 [1.31]; p < 0.001), and disease-specific quality of life (ASQoL: –2.51 [2.55] vs. control: –0.16 [2.26]; p < 0.001), without meaningful changes in the control group (Figure 3A). Furthermore, a significantly higher proportion of patients in the intervention group achieved an ASAS20 response compared to the control group (51% vs. 9%; p < 0.001), and the ASAS40 response rate was also markedly higher (23% vs. 3%; p < 0.001) (Figure 3B). No concerning safety signals were detected.
Conclusion: In this 12-week RCT involving axSpA patients receiving stable pharmacotherapy, the DTx Axia significantly improved disease activity, functional status, and disease-specific quality of life. These findings support the potential of Axia as a novel therapeutic option for patients with axSpA.
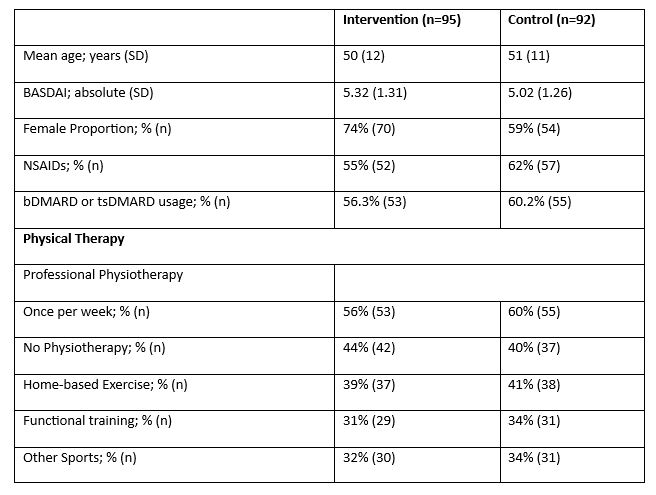 Baseline characteristics of the study population
Baseline characteristics of the study population
.jpg) Flowchart and trial design of the Bechterew-App Trial
Flowchart and trial design of the Bechterew-App Trial
.jpg) Results of the Bechterew-App Trial
Results of the Bechterew-App Trial
A) The intervention group showed improvements in mean BASDAI, BASFI and ASQoL
The analysis was conducted using ANCOVA, adjusted for baseline values and sex. ***: p < 0.001.
Abbreviations: ASQoL, Ankylosing Spondylitis Quality of Life Questionnaire; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index.
B) Efficacy of Axia assessed by ASAS20 and ASAS40 response criteria.
ASAS20 response was defined as an improvement of at least 20% and at least 1 unit (on a 0–10 scale) from baseline in three of four domains (spinal pain [BASDAI question 2], spinal inflammation [mean of BASDAI questions 5 and 6], physical function [BASFI], and patient global assessment by visual analogue scale [VAS]), with no worsening in the remaining domain (defined as a deterioration of ≥20% or ≥1 unit). ASAS40 response required an improvement of at least 40% and 2 units in three of the four domains without worsening in the fourth. ***: p < 0.001.
Abbreviations: ASAS, Assessment of Spondyloarthritis International Society; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index.
To cite this abstract in AMA style:
Strunz P, Heusinger T, Le Maire M, Fleischer A, Luetkens K, Possler P, Gernert M, Labinsky H, Leppich R, Schmieder A, Hammel L, Sperlich B, Fröhlich M, Schmalzing M. The Novel Digital Therapeutic Axia Improves Disease Activity, Functionality, and Quality of Life in Axial Spondyloarthritis Patients: Results from a Randomized Controlled Trial (Bechterew-App Trial) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-novel-digital-therapeutic-axia-improves-disease-activity-functionality-and-quality-of-life-in-axial-spondyloarthritis-patients-results-from-a-randomized-controlled-trial-bechterew-app-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-novel-digital-therapeutic-axia-improves-disease-activity-functionality-and-quality-of-life-in-axial-spondyloarthritis-patients-results-from-a-randomized-controlled-trial-bechterew-app-trial/
