Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II: Diagnosis and Prognosis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
A multi-biomarker disease activity (MBDA) score was developed to objectively measure disease activity for patients with rheumatoid arthritis (RA).1 The MBDA score is calculated by an algorithm using concentrations of 12 serum biomarkers and has been shown to track response to treatment with several DMARDs.2-4 We aimed to assess the ability of the MBDA score to track response to rituximab treatment in RA patients.
Methods:
Data were used from 3 cohorts (1 in the United Kingdom, 2 in the Netherlands) of RA patients treated with rituximab 1000 mg and methylprednisolone 100 mg at days 1 and 15. The MBDA score was assessed in serum samples at baseline (BL, n=57) and at 6 months (n=46). Wilcoxon signed-rank test was used to statistically compare the medians at BL and 6 months. Spearman’s rank correlation (r) was used to analyse relationships between BL and 6 month values and change (Δ) from BL to 6 months for MBDA score vs. the following endpoints: DAS28-ESR, DAS28-hsCRP, ESR, hsCRP and Health Assessment Questionnaire (HAQ). Logistic regression analysis with adjustment for age, sex, smoking, ACPA and RF was used to assess the association between ΔMBDA score and non-response, using EULAR response categories at 6 months. p <0.05 was considered statistically significant.
Results:
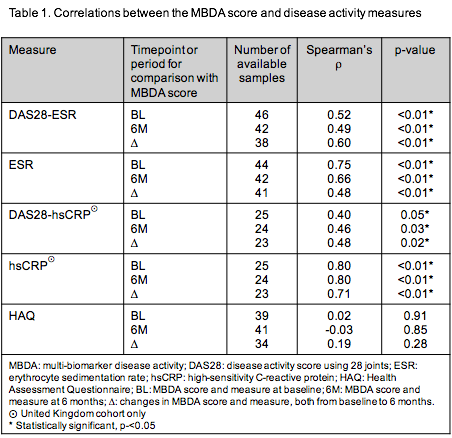
At baseline the median MBDA score and DAS28-ESR were 56.0 (range 16.0-84.0) and 6.2 (range 2.5-8.4), respectively. The improvement in both scores after 6 months was statistically significant (both p<0.01). MBDA score correlated with DAS28-ESR, DAS28-hsCRP, ESR and hsCRP at BL and 6 months, respectively (Table 1). ΔMBDA score from BL to 6 months correlated with changes in these measures. Spearman’s correlation for ΔMBDA score vs. ΔDAS28-ESR was r=0.60, p<0.01 (Table 1). DMBDA score also correlated with EULAR non-response (n=38), with adjusted OR=0.91 (95% CI=0.84–0.99, p=0.02), which corresponds to an OR of 0.39 for every 10-unit decrease in MBDA score. Correlations were not observed between MBDA scores or ΔMBDA score and the corresponding HAQ measurements (Table 1).
Conclusion:
We have shown, for the first time, that the MBDA score tracked disease activity in RA patients treated with rituximab and that change in MBDA score reflected the degree of treatment response.
References
1 Centola M et al. Development of a MBDA Test for RA. PLoS One. 2013;8(4).
2 Bakker MF et al. Performance of a multi-biomarker score measuring RA disease activity in the CAMERA tight control study. Ann Rheum Dis. 2012;71(10):1692–7.
3 Jurgens MS et al. The MBDA Test for Assessing Response to Treatment Strategies Using Methotrexate with or without Prednisone in the CAMERA Trial-II. ISBN: 978-94-6259-393-0. 2014;81-98.
4 Hirata S et al. A MBDA score tracks clinical response consistently in patients with RA treated with different anti-tumor necrosis factor therapies: A retrospective observational study. Mod Rheumatol. 2015;25(3):344–9.
To cite this abstract in AMA style:
Roodenrijs NM, de Hair MJ, Wheater G, Elshahaly M, Tekstra J, Teng YKO, Lafeber FP, Hwang CC, Liu X, Sasso EH, van Laar J. The Multi-Biomarker Disease Activity Score Tracks Response to Rituximab Treatment in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-multi-biomarker-disease-activity-score-tracks-response-to-rituximab-treatment-in-rheumatoid-arthritis-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-multi-biomarker-disease-activity-score-tracks-response-to-rituximab-treatment-in-rheumatoid-arthritis-patients/