Session Information
Date: Tuesday, November 15, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment - Poster III: Biomarkers and Nephritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical trials in lupus nephritis have often been designed to demonstrate renal response (or remission) following therapy based on categorical remission endpoints (often no remission, partial and complete) derived from laboratory measures of renal function and activity. The clinical relevance of these remission categories has been questioned. The primary objective of this study (WEUKBRE6068) was to compare long-term renal survival in patients with complete (CR), partial (PR) or no remission, as defined by criteria used in the Aspreva Lupus Management Study (ALMS) (NCT00377637) and Belimumab International Lupus Nephritis Study (BLISS-LN) (NCT01639339) with the modification of excluding urinary sediments (mBLISS-LN and mALMS), assessed at 24 months following positive lupus nephritis biopsy.
Methods: A retrospective analysis of the prospective Hopkins Lupus Cohort was conducted. Eligible patients had systemic lupus erythematosus (SLE) per revised American College of Rheumatology (ACR) or Systemic Lupus International Collaborating Clinics (SLICC) criteria plus biopsy record of ISN class III, IV, V or mixed lupus glomerulonephritis. The primary endpoint was renal survival (survival without end-stage renal disease (ESRD) or mortality). The primary exposure was remission status (CR, PR or no remission) by mBLISS-LN and mALMS criteria at 24 months post biopsy date. Survival analysis (Kaplan-Meier plots with log-rank test and Cox Proportional Hazards regression) was used to describe subsequent event rates and assess the association between renal survival and remission status at 24 months.
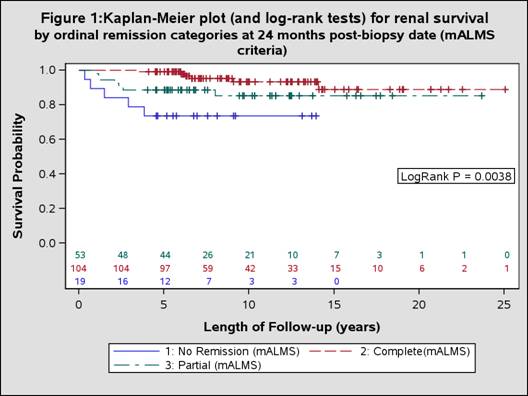
Results: We identified 176 SLE patients with lupus nephritis. At 24 months post biopsy date, more patients met mALMS remission criteria (CR = 59.1%, PR = 30.1%) than met mBLISS-LN criteria (CR = 40.9%, PR = 16.5%). During subsequent follow-up, 18 patients developed ESRD or died. The Kaplan-Meier plots suggested patients with no remission at 24 months post biopsy date were more likely than those with PR or CR to develop the outcome by both mALMS (p=0.0038) (Figure 1) and mBLISS-LN (p=0.0097) (Figure 2) criteria. Based on Cox regression models adjusted for key confounders, those in CR by both mBLISS-LN (HR 0.254, p=0.0176) and mALMS criteria (HR 0.228,p=0.0246) were significantly less likely to experience ESRD/mortality than those not in remission. Similarly, those in PR were less likely to experience ESRD/mortality (mBLISS-LN HR 0.141, p=0.0599; mALMS HR 0.575,p=0.3727)
Conclusion: Renal remission status at 24 months following lupus nephritis is associated with long-term renal survival.
To cite this abstract in AMA style:
Davidson J, Fu Q, Ji B, Rao S, Roth D, Magder LS, Petri M. The Long-Term Clinical Outcomes of Lupus Nephritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-long-term-clinical-outcomes-of-lupus-nephritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-long-term-clinical-outcomes-of-lupus-nephritis/