Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Co-morbid fibromyalgia has been shown to be an important influence on non-response in patients with some inflammatory arthritides. Around 1 in 5 patients with PsA have been estimated to have co-morbid fibromyalgia. Here we examine to what extent meeting criteria for fibromyalgia or reporting symptoms typical of fibromyalgia is an important determinant of non-response to a biologic or targeted synthetic (b/ts) DMARD, taking into account other predictors of non-response.
Methods: Data included were from BSR-PsA (January 2023 download), a prospective registry of patients with PsA who meet CASPAR criteria, recruited across Great Britain. In this analysis we include those newly commencing a bDMARD/tsDMARD (having not previously taken the same agent). The association between patient characteristics and treatment response (PsARC) was determined by logistic regression. The fibromyalgia polysymptomatic distress score (FMness) (Model1) or fibromyalgia 2016 (FM2016) criteria (Model2) was forced into the model and then other predictors of poor outcome which improved the fit of the model were determined using a stepwise procedure. There were 12 candidate variables across social/demographic/economic, clinical and patient-reported outcome domains.
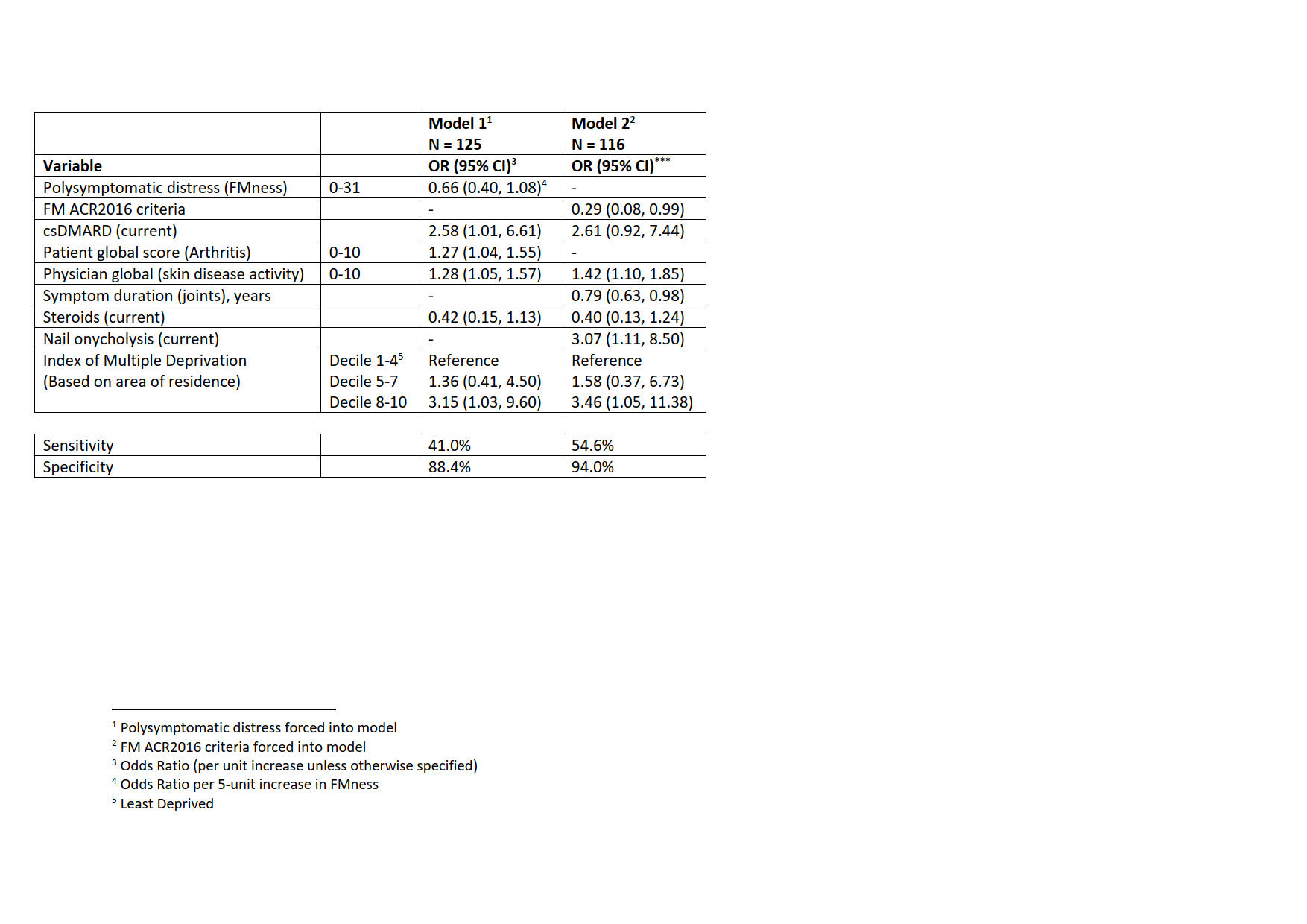
Results: 148 patients were included with median age 52 years, 29% male, 52% obese with a median FMness score of 12 and 29% met FM criteria. They had a median disease duration of 7 years, a median Quality of Life score (PsAQoL) of 12, physician global (musculoskeletal and skin) of 5.1, Disease Activity in Psoriatic Arthritis (DAPSA) 34 and 57% had ≥1 comorbidity. Follow-up was at a median of 6 months (IQR 4.9-6.7) at which point 28% fulfilled PsARC. Multivariable regression models showed that patients meeting FM2016 (OR 0.3 95% CI (0.1, 0.99) or with fibromyalgia symptoms (OR 0.7, 95% CI (0.4, 1.1) per 5 unit increase in FMness (Table) were less likely to meet PsARC after accounting for other predictors of response accepted into the model (living in a less deprived area, concurrent therapies, shorter symptom duration, nail onycholysis, poorer physician and patient global measures).
Conclusion: PsA patients with features of fibromyalgia, particularly those meeting current fibromyalgia criteria, commencing b/tsDMARDs, are considerably less likely to meet response criteria, independently of other predictors of outcome. They are likely to benefit from additional management specifically targeted to their fibromyalgia symptoms.
To cite this abstract in AMA style:
Macfarlane G, Rotariu O, Lembke S, Sunzini F, Jones G, Basu N. The Influence of “Fibromyalgia-ness” on Treatment Response Amongst Patients with Psoriatic Arthritis (PsA). Results from the British Society for Rheumatology Psoriatic Arthritis Register (BSR‐PsA) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-influence-of-fibromyalgia-ness-on-treatment-response-amongst-patients-with-psoriatic-arthritis-psa-results-from-the-british-society-for-rheumatology-psoriatic-arthritis-regist/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-influence-of-fibromyalgia-ness-on-treatment-response-amongst-patients-with-psoriatic-arthritis-psa-results-from-the-british-society-for-rheumatology-psoriatic-arthritis-regist/