Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose :
The impact of Body mass Index (BMI) on efficacy of TNF blocking therapy is an important question, as adipose tissue appears to have an immunomodulating effect (Tilg et al 2006), and may adversely impact treatment response in patients receiving TNF blocking therapy (Gremese et al 2013). The aim of this study is to ascertain the impact of BMI on the efficacy of TNF blocking therapy with either adalimumab or etanercept for the treatment of RA.
Methods :
At the outpatient clinic in Amsterdam, the Netherlands, 853 consecutive RA patients were included who started with TNF blocking therapy adalimumab (411) or etanercept (442), and of whom baseline BMI and follow-up data for at least 16 weeks was available. Adalimumab was dosed at 40 mg fortnightly and etanercept at 50 mg weekly for all patients, regardless of body weight.
Results:
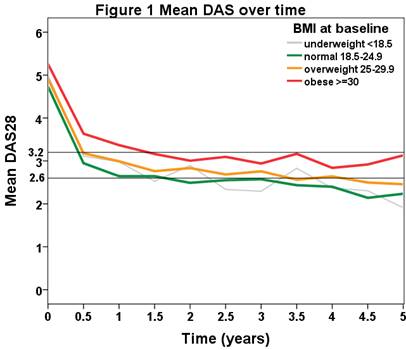
The average BMI was 26.0 (SD: 5.2, range 14-50). The baseline DAS was high at 4.9 (SD: 1.3). Mean age was 54 years (SD: 13) and 682 (80%) were female. Median disease duration was 7 years (IQR: 3-16). Median follow-up was 2.1 years (IQR 0.7-4.4 years), 2337 patient years total. Nearly half (407, 48%) of patients were of healthy weight according to the WHO criteria (BMI 18.5-25), 257 (30%) of the patients were overweight, and another 168 (20%) were obese (BMI of 25-30, and >30 respectively). Few patients (21, 2%) were underweight (BMI<18.5). Overall, the patients with healthy weight responded best to TNF blocking therapy, followed by the overweight and the underweight groups. The group who performed worst was the obese group (see Figure 1). After one year the overweight group had a significantly higher DAS (28 joints) than the normal weight group (3.0 (1.3) vs. 2.6 (1.2), p= 0.005), and the obese group did significantly worse than either (3.4 (1.4), p=0.000 (vs. normal) and 0.026 (vs. overweight)). The DAS at one year of the underweight group did not differ significantly from any other weight group. When comparing the individual components of the DAS for obese vs. normal weight RA patients, both tender joint score and BSE were elevated at baseline and 52 weeks, and patient global assessment was only significantly higher at 52 weeks for the obese group. Swollen joint score was not significantly different at baseline or 52 weeks.
Conclusion:
Our findings support the hypothesis that adipose tissue has an impact on treatment outcome of TNF-blockers in RA.. This is important as it may have implications for other immune-mediated inflammatory conditions treated with TNF blocking therapy.
In addition, as adalimumab and etanercept are given in a standard dose, regardless of body weight, it may be that for obese patients a better response could be achieved with an increased dose. This could be an important avenue for further study, and eventually leading to better treatment outcomes of obese RA patients.
Disclosure:
I. M. Visman,
None;
I. A. M. van den Oever,
None;
C. L. M. Krieckaert,
None;
G. Wolbink,
None;
M. T. Nurmohamed,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-influence-of-body-mass-index-on-the-efficacy-of-tumor-necrosis-factor-blocking-therapy-for-rheumatoid-arthritis/