Session Information
Date: Sunday, November 8, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Comorbidities
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Although there is abundant literature on healthcare utilization in SLE patients, the impact of disease activity in SLE patients is not well understood.
To quantify the impact of disease activity, as measured by SLEDAI score and drug burden, in SLE patients on health care resource utilization (HCRU), health related quality of life (HRQoL) and work productivity (WP).
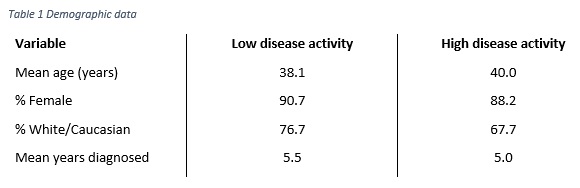
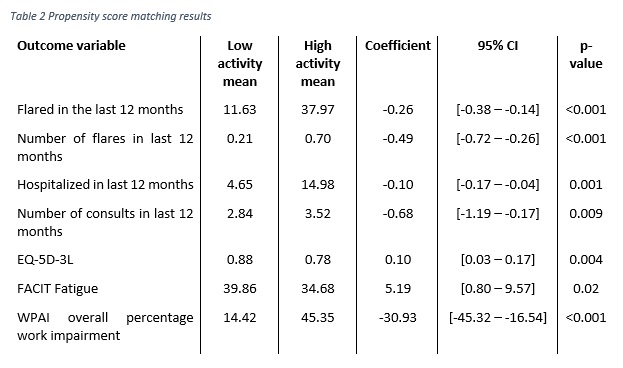
Methods: Data were collected from a cross-sectional survey of 300 rheumatologists completing 752 patient record forms in US and EU5 from the Adelphi Real World 2010 & 2013 Lupus Disease Specific Programmes (DSP). Physicians were asked to complete patient record forms (PRFs) for the next 5 prospectively consulting SLE patients; the same patients were asked to complete patient self-completion (PSC) forms describing how SLE affected them. PRFs collected data pertaining to the patient’s diagnosis, disease history, current clinical outcomes, treatment and management history. PSCs focused on similar data collection and included patient reported outcome measures (PROs). Propensity score matching was used to assess differences in HCRU and PRO scores between SLE patients who had a low disease activity and those who had a high disease activity. Low disease activity was defined as a SLEDAI score of ≤4, a steroid dose of < 7.5mg/day, and not on immunosuppressant or biologic. High disease activity was a SLEDAI score of >4, or on an immunosuppressant, biologic, or steroid dose of >7.5mg/day. Patients were matched on age, sex, and ethnicity.Results: Data was extracted from 752 PRFs, and 388 PSCs. Using the estimated propensity score each low disease activity patient (n=43) was matched with a high disease activity patient (n=709). Using 1:1 matching, with replacement and allowing for ties, matching resulted in 257 high disease activity patients being used as matches for 43 low disease activity patients. Demographic data are reported in Table 1. Patients with a low disease activity were significantly less likely to be currently flaring, lower number of flares in last 12 months, less likely to have been hospitalized in the last 12 months, had fewer consultations in the last 12 months, reported better HrQoL (EQ5D), more favourable levels of fatigue (FACIT), and lower work impairment (WPAI). (Table 2).
Conclusion: Systemic lupus erythematosus patients with lower levels of disease activity are less burdensome to the healthcare system and experience a significantly better HRQoL and lower levels of productivity impairment. There is a need to establish a universal definition of low disease activity as a treatment goal to benefit patient quality of life and reduce HCRU.
To cite this abstract in AMA style:
Touma Z, Hoskin B, Atkinson C, Bell D, Pike J, Lofland J, Berry P, Karyekar C, Costenbader K. The Impact of High Disease Activity as Measured by SLEDAI and Drug Burden on Healthcare Utilization, Quality of Life and Work Productivity in Systemic Lupus Erythematosus Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-impact-of-high-disease-activity-as-measured-by-sledai-and-drug-burden-on-healthcare-utilization-quality-of-life-and-work-productivity-in-systemic-lupus-erythematosus-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-high-disease-activity-as-measured-by-sledai-and-drug-burden-on-healthcare-utilization-quality-of-life-and-work-productivity-in-systemic-lupus-erythematosus-patients/