Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with rheumatoid arthritis (RA) often experience substantial pain despite treatment, and consider pain control an important treatment outcome. This post hoc analysis of the FINCH studies assessed specific effects of filgotinib (FIL) on pain and its relationship with efficacy in pts with RA.
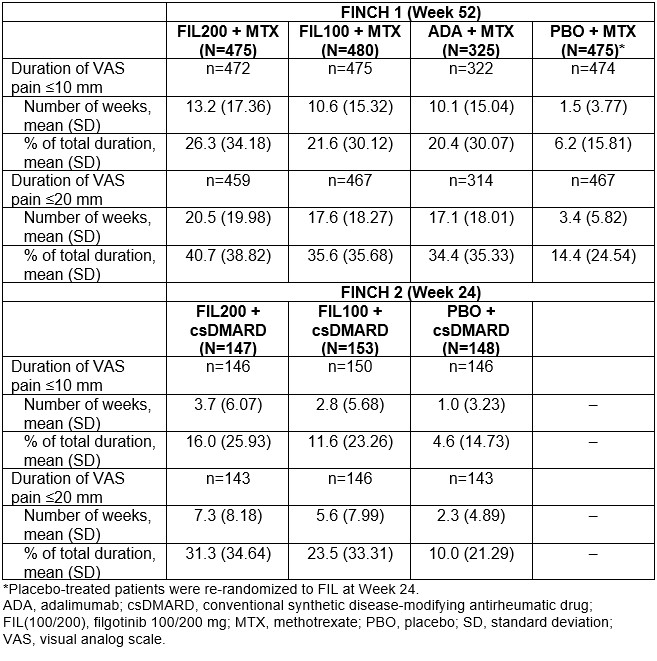
Methods: FINCH 1–3 (NCT02889796, NCT02873936, NCT02886728) were Phase 3, randomized, double-blind trials of FIL 100 mg and 200 mg (FIL100/200). In FINCH 1, pts with an inadequate response (IR) to methotrexate (MTX) received FIL, adalimumab (ADA) or placebo (PBO) + MTX for 52 weeks. In FINCH 2, pts with an IR to bDMARDs received FIL or PBO + csDMARDs for 24 weeks. In FINCH 3, MTX-naïve pts received FIL ± MTX or MTX for 52 weeks. For each treatment group, pts reported pain on a 100-mm visual analog scale (VAS). VAS pain ≤ 20 mm indicated health status was not negatively affected by pain; ≤ 10 mm reflected limited to no pain.1 Time to first VAS pain ≤ 10 mm was assessed. The duration of the study period during which VAS pain was ≤ 20 mm or ≤ 10 mm and the proportion of pts who achieved remission (DAS28-CRP < 2.6 or CDAI ≤ 2.8) at Week 24 was evaluated. Of pts who achieved DAS28-CRP or CDAI remission, the proportion who also reported VAS pain ≤ 20 mm or ≤ 10 mm was determined.
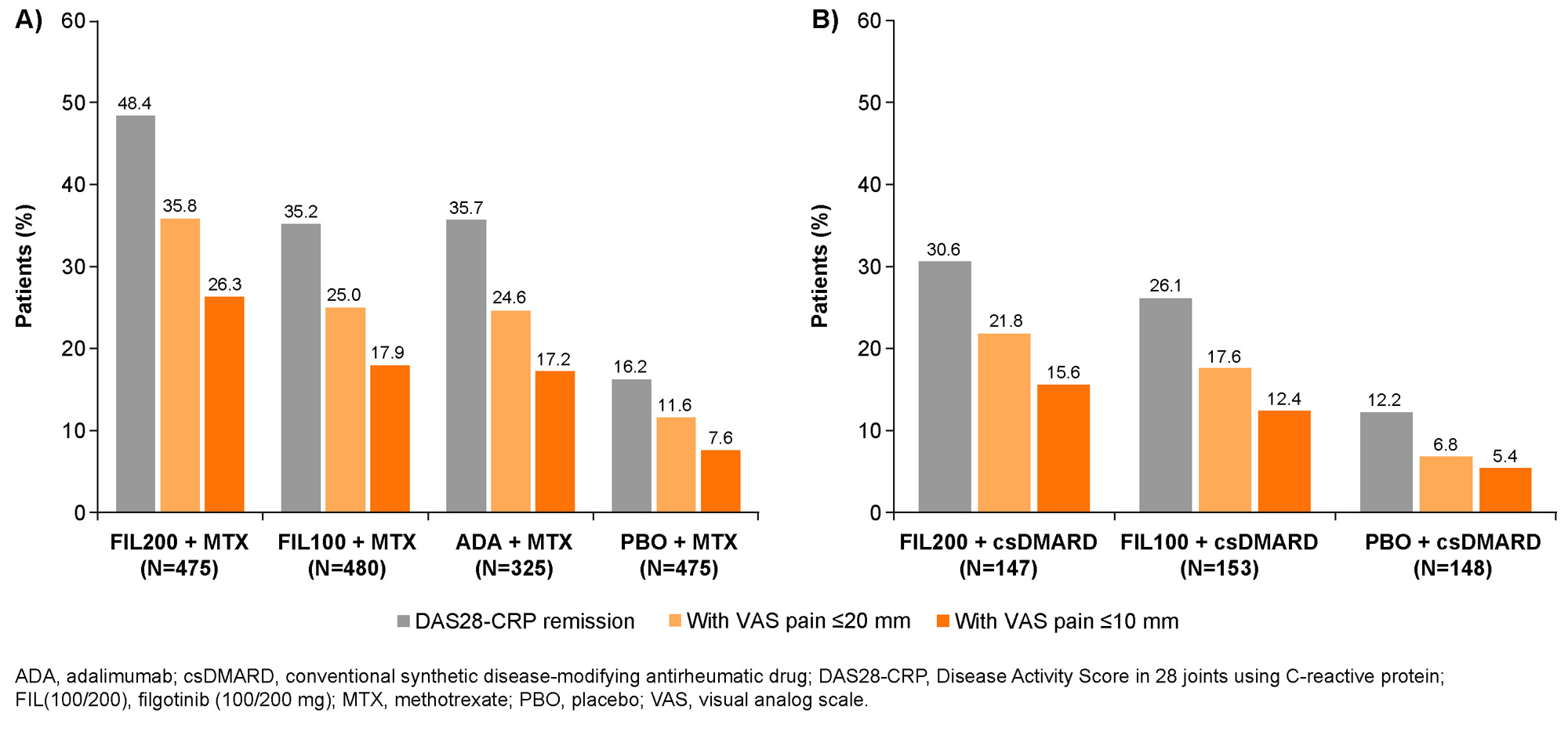
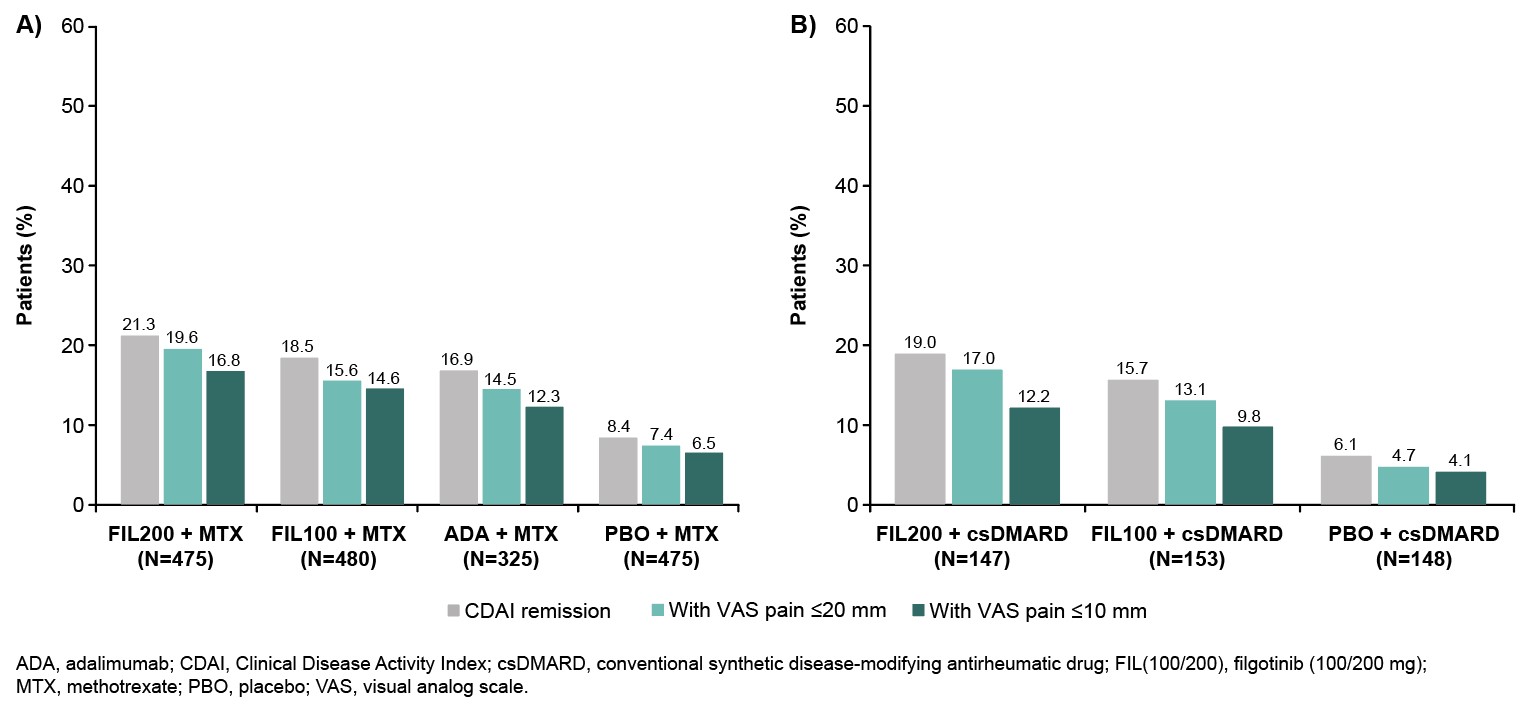
Results: In FINCH 1, there was a higher probability of achieving VAS pain ≤ 10 mm with FIL200, vs ADA + MTX or PBO + MTX; responses were better or comparable with FIL100 vs other treatments. Similar findings were seen in FINCH 2 and 3. In FINCH 1, the time during which VAS pain score was ≤ 20 mm or ≤ 10 mm was greatest in the FIL200 group, and comparable between the FIL100 and ADA groups (Table). The duration of time under each pain threshold was greater in each FIL group vs PBO in FINCH 2 (Table) and in each FIL group vs MTX in FINCH 3. In FINCH 1, the proportion of pts achieving DAS28-CRP remission was greater with FIL200 + MTX (48.4%) and comparable for FIL100 + MTX (35.2%) vs ADA + MTX (35.7%; Figure 1A). The proportion of pts who achieved VAS pain ≤ 20 mm and ≤ 10 mm in addition to DAS28-CRP remission was 35.8% and 26.3%, respectively, in the FIL200 + MTX group, vs 24.6% and 17.2% in the ADA + MTX group (Figure 1A). In FINCH 2, a greater proportion of pts in the FIL groups achieved DAS28-CRP remission and pain responses in addition to DAS28-CRP remission vs the PBO group (Figure 1B). Findings were similar when CDAI remission was assessed for FINCH 1 and 2 (Figure 2A and B). In FINCH 3, the proportion of pts to achieve remission and pain responses in addition to remission was greater in the FIL vs MTX groups; e.g. DAS28-CRP remission and VAS pain ≤ 10 mm was achieved by 23.3% of pts on FIL200, 33.2% on FIL200 + MTX, 27.5% on FIL100 + MTX and 14.9% on MTX.
Conclusion: Across the studies, duration of threshold pain responses achieved over the observation periods was greater with FIL vs comparators. In FINCH 1, FIL200 had a particularly favorable impact when pain response and remission were assessed together; results were comparable between FIL100 and ADA. Similar findings were seen with FIL vs PBO and MTX in FINCH 2 and 3, respectively. These findings suggest that JAK1 inhibition may offer potential added value with respect to patient-reported pain as well as treat-to-target goals.
Reference:
1. Taylor PC, et al. J Clin Med 2019;8:831
To cite this abstract in AMA style:
Taylor P, Kavanaugh A, Nash P, Pope J, Pongratz G, Fautrel B, Alten R, Hasegawa K, Rao S, de Vries D, Stiers P, Watson C, Westhovens R. The Impact of Filgotinib on Disease Activity Outcomes with Concomitant Pain Control in the Phase 3 FINCH Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-filgotinib-on-disease-activity-outcomes-with-concomitant-pain-control-in-the-phase-3-finch-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-filgotinib-on-disease-activity-outcomes-with-concomitant-pain-control-in-the-phase-3-finch-studies/