Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The detection of antibodies to cyclic citrullinated peptides (anti-CCP) may occur long before the onset of rheumatoid arthritis (RA) symptoms.
Several versions of CCP antigen peptide mixtures exist in commercial laboratory testing and are labelled CCP 2, CCP 3.0, CCP 3.1. However, despite the sequential numbering of these antigens, newer generations are not necessarily associated with improved clinical performance [Mathsson-Alm et al., 2018].
The objective of this study was to perform a systematic literature review and meta-analysis aimed at comparing the diagnostic accuracy of the most accurate anti-CCP 2 test as identified in the most recent systematic literature review and meta-analysis published on CCP tests [Mathsson-Alm et al., 2018], with anti-CCP 3.0 and CCP 3.1 tests for the diagnosis of RA, with a focus on early RA.
Methods: Our systematic literature review identified studies from 2004-2018. Estimates for pooled sensitivity, pooled specificity, Area Under the Curve (AUC), Diagnostic Odds Ratios (DOR), positive/negative likelihood ratio (LR+/LR-) were calculated; sub-analyses stratified by study design (single-gated, which are cross-sectional studies, or double-gated studies, which are case-control studies), by test generation, and by early RA (defined as symptoms durarion less than two years).
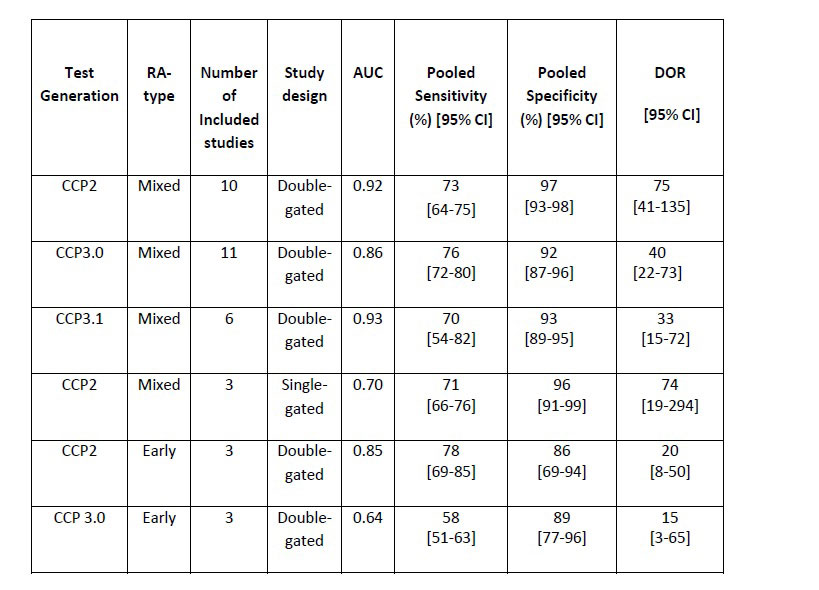
Results: Out of 5,074 papers, 113 met the inclusion criteria reporting the diagnostic accuracy of anti-CCP tests, and included 84 studies from early RA patients.
The results displayed in the Table show that the CCP 2 test has a stable performance across study designs for undifferentiated RA. As expected, when used in single-gated study designs, the test is characterized by a slightly lower sensitivity (non significant difference). It is worth noting here that single-gated studies are more representative of the scenario in which the tests would be used in practice to diagnose RA. The two CCP 3 tests are characterized by overall good and comparable performance, CCP 3.1 being characterized by a slightly lower sensitivity.
In early RA it was not possible to stratify the analysis by study design due to lack of articles passing the inclusion criteria. Moreover, not enough data was found in the literature for the test CCP 3.1 for this RA sub-type, so the comparison is limited to two tests only. The meta-analysis results show that the CCP 2 test is characterized by significantly higher sensitivity (p< 0.05) and by similar specificity compared to the test CCP 3.1.
Conclusion: The meta-analysis highlights that the CCP 2 test considered is in general characterized by higher diagnostic accuracy, demonstrating the greatest combination of sensitivity and specificity for RA, in undifferentiated RA as well as in early RA. These results suggest that using CCP 3.0 or CCP 3.1 assays may result in the misclassification of some RA patients.
To cite this abstract in AMA style:
Mascialino B, Mathsson-Alm L, Poorafshar M, Tarrant T. The Generation of Anti-CCP Tests Affects Diagnostic Accuracy in Rheumatoid Arthiritis: A Systematic Literature Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-generation-of-anti-ccp-tests-affects-diagnostic-accuracy-in-rheumatoid-arthiritis-a-systematic-literature-review-and-meta-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-generation-of-anti-ccp-tests-affects-diagnostic-accuracy-in-rheumatoid-arthiritis-a-systematic-literature-review-and-meta-analysis/