Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Several of the most commonly prescribed anti-rheumatic medications for women with rheumatic disease are known teratogens, posing a risk for pregnancy loss and birth defects if taken during pregnancy. Despite the importance of avoiding pregnancy while taking a teratogenic medication, within the U.S. an estimated 10-15% of pregnancies to women with rheumatic disease are conceived while the mother is using a teratogen. We sought to understand the frequency of contraception documentation for women with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) in a large U.S. electronic-health-record (EHR) based registry, and to identify disparities by teratogen prescription and patient race and ethnicity.
Methods: RISE (Rheumatology Informatics System for Effectiveness) is a national EHR-enabled rheumatology registry that passively collects data on all patients seen by participating practices. As of 2018, RISE held validated data from 1,113 clinicians in 226 practices, representing an estimated 32% of the U.S. clinical rheumatology workforce. Women of childbearing age (18-45 years) in 2018 and at least 2 visits with ICD-9 or -10 diagnosis codes for SLE or RA (at any time) were assessed for anti-rheumatic medication prescriptions and contraception documentation within a structured data field. The proportion of patients with documented contraception was compared by teratogen prescription and patient age and race/ethnicity.
Results: In 2018, 110,359 women between the ages of 18-45 had at least 1 visit: 9,826 with SLE and 19,009 with RA. Overall, 8.9% had any contraception documented.
More women with RA than SLE had documentation of contraception (SLE 7.9%, RA 9.4%, p< 0.001). Among SLE patients, the rate of contraception documentation was similar whether or not the woman was prescribed a teratogenic anti-rheumatic medication. Among women with RA, however, contraception documentation was greater among women prescribed a teratogen than those prescribed only pregnancy-compatible medications (10.3% vs 9.1%, p=0.02).
The rate of contraception documentation was higher in white women (SLE 9.4%, RA 10.9% (see figure) compared to Hispanic women (SLE 5.7%, p=0< 0.001; RA 5.4%, p< 0.001) or black women (SLE 7.0, p< 0.001; RA 6.3, p< 0.001).
For women with SLE and RA, 1.5-1.7% of women used highly effective contraception (i.e., tubal ligation, intrauterine device, subdermal implant) and 5.0-5.6% had documentation of other forms of effective contraception (i.e., pill, patch, ring, or injection).
Conclusion: We found large gaps in contraception documentation within the RISE registry. While roughly 70% of women in the U.S. report using contraception, contraception was documented in < 10% of eligible women in RISE. While these data likely underestimate contraception use, they also highlight that many rheumatologists do not have a systematic approach to collecting and recording this information in the EHR. The racial disparities noted within this study require additional study, and may suggest that implicit bias plays a role in ascertaining and recording contraception use within the RISE Registry.
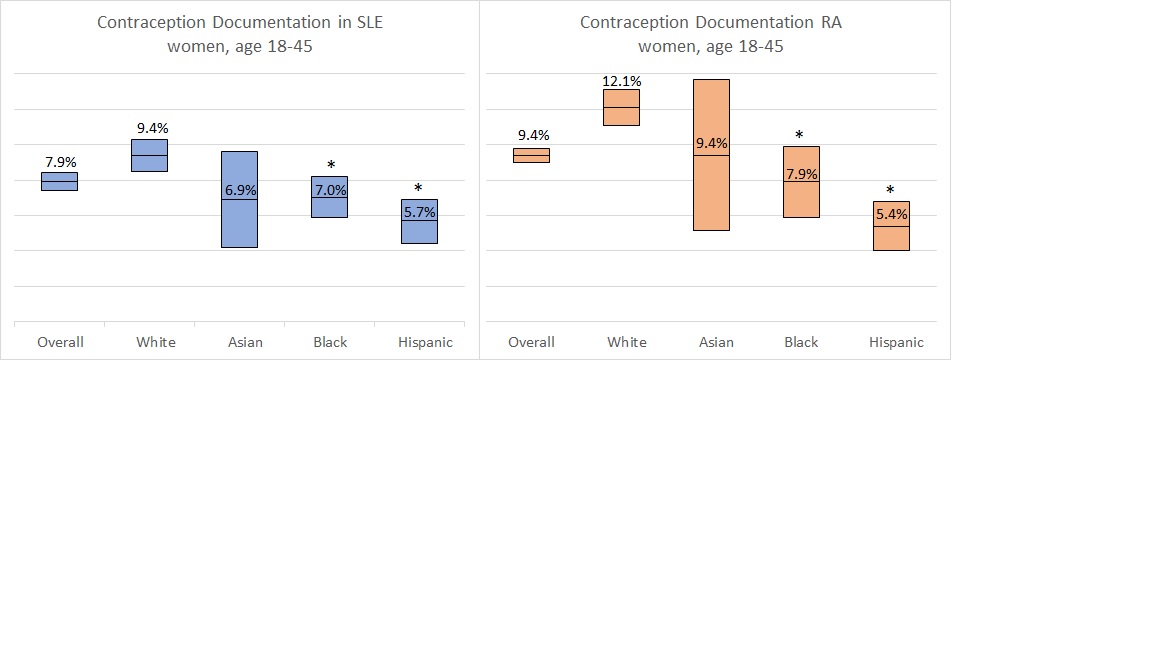 Contraception documentation among women with SLE and RA by race and ethnicity (mean noted, range is 95% CI; *p-value < 0.001 compared to white women)
Contraception documentation among women with SLE and RA by race and ethnicity (mean noted, range is 95% CI; *p-value < 0.001 compared to white women)
To cite this abstract in AMA style:
Clowse M, Li J, Eudy A, Birru Talabi M, Schmajuk G. The Frequency of Contraception Documentation in Women with Lupus and Rheumatoid Arthritis Within the RISE Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-frequency-of-contraception-documentation-in-women-with-lupus-and-rheumatoid-arthritis-within-the-rise-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-frequency-of-contraception-documentation-in-women-with-lupus-and-rheumatoid-arthritis-within-the-rise-registry/
