Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: In this study, it was aimed to evaluate the long-term drug survival, efficacy and safety of infliximab biosimilar CT-P13 in Turkish patients diagnosed with ankylosing spondylitis (AS) in clinical practice. The primary outcome is the assessment of drug retention (ie, time until treatment discontinuation or switching to another biological therapy) and disease activity scores in Turkish patients with AS. Additional results evaluated its efficacy and safety.
Methods: Data were collected from TURKBIO database. CT-P13 efficacy was assessed using standard disease activity parameters, while drug-related serious adverse events (AEs) were recorded.
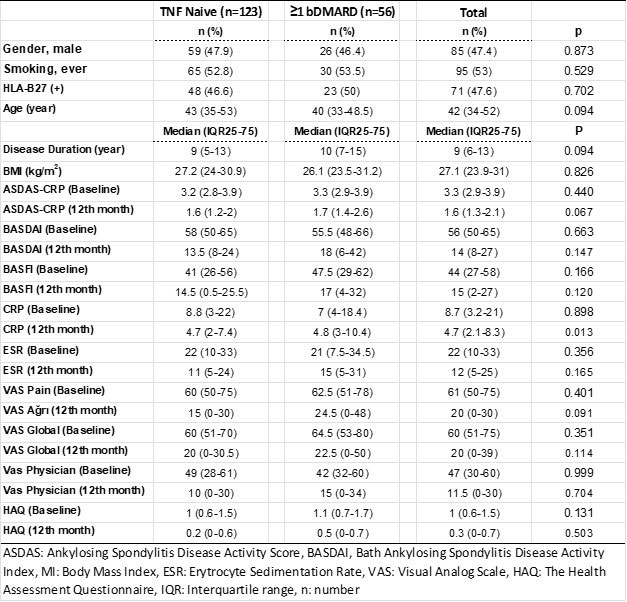
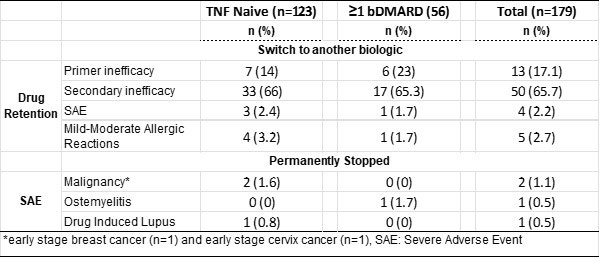
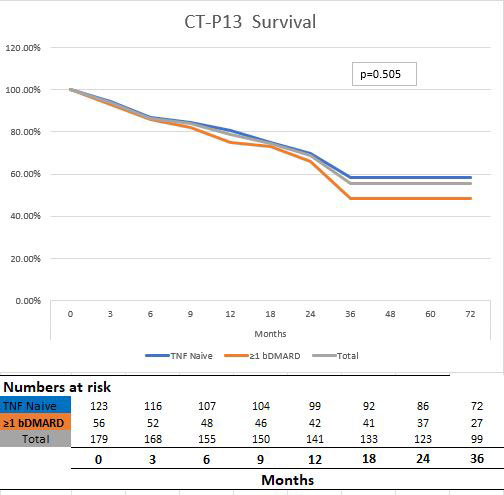
Results: Between December 2014 and December 2021, 179 patients with AS treated with CT-P13 were enrolled. Of these, 123 (68.7%) were using CT-P13 as first-line therapy. The mean duration of treatment was 3.5 years (Table 1). After 3 years of follow-up, CT-P13 drug survival rates in the general patient population were 58.6% and 48.2% in naive and ≥1 TNFi switch patients, respectively (Table 2 and Graphic 1). While the most common reason for treatment changes was treatment failure, AEs were the other most common reason for discontinuation of treatment. Disease activity decreased significantly compared to baseline after initiation of CT-P13 therapy and remained stable for a long time. In a follow-up of more than 5 years, treatment change was required due to severe allergic reaction in 4 patients (2.2%) and moderate allergic reaction in 5 patients (2.7%). During the treatment, TNFi treatment was completely discontinued because cancer developed in 2 (1.1%) patients, osteomyelitis in 1 (0.5%) and drug-related lupus in 1 (0.5%) (Table 2).
Conclusion: In this real-life data study, CT-P13 therapy achieved promising drug-survival rates with reasonable long-term efficacy and safety in naïve and ≥1 TNFi-refractory Turkish AS patients.
To cite this abstract in AMA style:
Uslu S, Gulle S, Can G, Senel S, Capar S, dalkilic H, Akar S, Koca S, Tufan A, Yazici A, Yilmaz S, Inanc N, Birlik M, Solmaz D, Cefle A, Goker B, Yolbas S, Krough N, Yilmaz N, Erten S, Bes C, Soysal O, Ozturk M, Haznedaroglu S, Yavuz S, Direskeneli H, Onen F, Sari I. The Efficacy and Safety of CT-P13 as First-line and Subsequent-line Therapy in Patients with Ankylosing Spondylitis: Real-life Data from TURKBIO Cohort [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-efficacy-and-safety-of-ct-p13-as-first-line-and-subsequent-line-therapy-in-patients-with-ankylosing-spondylitis-real-life-data-from-turkbio-cohort/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-efficacy-and-safety-of-ct-p13-as-first-line-and-subsequent-line-therapy-in-patients-with-ankylosing-spondylitis-real-life-data-from-turkbio-cohort/