Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Expressed in synovial fluid mononuclear and other inflammatory cells, peptidyl arginine deiminase (PAD) catalyzes post-translational citrullination of synovial proteins in a calcium (Ca2+)-dependent manner. Prior reports have demonstrated that post-translational modification of proteins with malondialdehyde-acetaldehyde (MAA) are also common in the RA synovium where they co-localize with citrullinated antigen. However, little is known about the effects of MAA modified proteins on PAD expression or intracellular Ca2+ concentrations. The purpose of this study was to examine the intracellular Ca2+ levels, and the PAD-4 mRNA and protein levels in macrophages following treatment with a MAA-modified antigen.
Methods: THP-1 human macrophage cells were treated with unmodified fibrinogen (Fib) as a negative control or MAA-modified fibrinogen (Fib-MAA) for 24 hours. Cells were washed and incubated with a Fluo-4 AM calcium-binding protein for 30 minutes and subjected to live cell imaging on a 710 Ziess confocal microscope. Images were acquired in 10-second intervals for 120 seconds and analyzed using Image J. RTPCR was performed on the cells to determine the expression of PAD-4 as well as the calcium binding proteins Inositol 1,4,5-triphosphate receptor-3 (ITPR3) and calcium/calmodulin-dependent protein kinase (CAMKK)-2. THP-1 cells were subjected to Western Blot for protein expression of PAD-4 using an anti-PAD antibody. Group differences were examined using ANOVA with a post-hoc test to account for multiple comparisons.
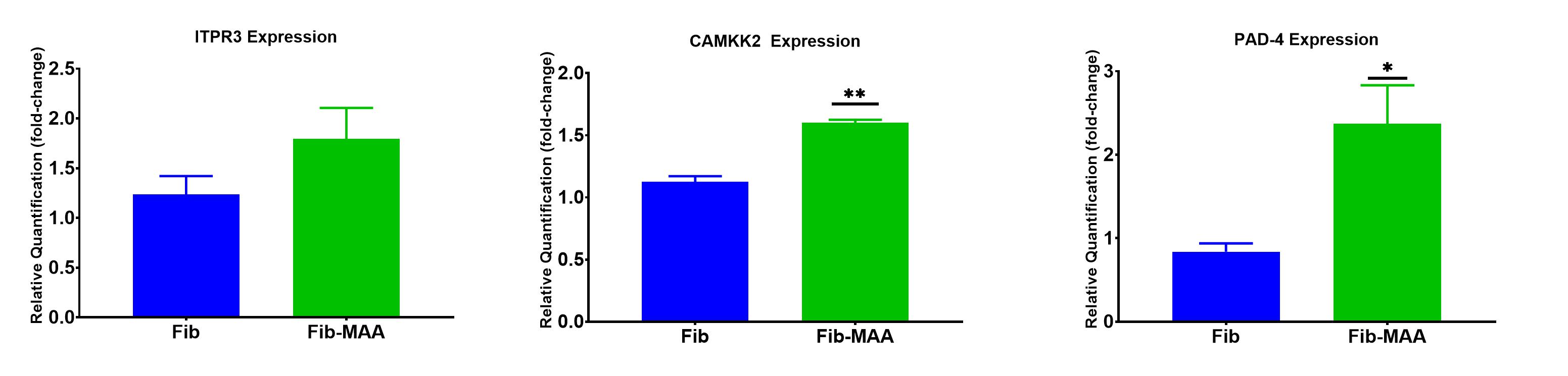
Results: Human macrophages incubated with Fib-MAA significantly increased (p< 0.001) the Ca2+ influx compared to unmodified Fib (Figure 1). Exposure to Fib-MAA had no meaningful impact on ITPR3 expression (p >0.05) (Figure 2). In contrast, CAMKK2 expression was significantly (p< 0.001) increased in macrophages stimulated with Fib-MAA compared to cells stimulated with unmodified Fib. Likewise, PAD-4 mRNA expression was significantly (p< 0.01) increased in only the Fib-MAA vs. native Fib. In contrast to the mRNA expression, Western Blot revealed a decrease in PAD-4 protein expression for Fib-MAA vs. unmodified Fib, suggesting protein depletion (Figure 3).
Conclusion: The increase in Ca2+ influx following treatment with a MAA-modified antigen provides a critical element needed for PAD-mediated protein citrullination in human macrophages, while the observed increase in PAD-4 expression provides the requisite catalyst. The lack of effect seen on ITPR3 (which promotes intracellular movement from the endoplasmic reticulum) suggests that the overall increase likely stems from extracellular sources. The increased expression of CAMKK2 following Fib-MAA exposure could account for changes in cellular activity such as cell differentiation, changes in cell cycle and/or cytoskeletal architecture even though the source of calcium is different. Taken together, these data for the first time implicate MAA-modified proteins as promoters of macrophage-mediated citrullination, a process that is critical in RA pathogenesis and that appears to be dependent on increases in both Ca2+ influx and increased PAD expression.
To cite this abstract in AMA style:
Gerber A, Duryee M, Aripova N, England B, O'Dell J, Mikuls T, Thiele G. The Effects of MAA-Modified and/or Citrullinated Proteins on Calcium Influx and Peptidyl Arginine Deiminase (PAD) Expression in Macrophage [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-effects-of-maa-modified-and-or-citrullinated-proteins-on-calcium-influx-and-peptidyl-arginine-deiminase-pad-expression-in-macrophage/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effects-of-maa-modified-and-or-citrullinated-proteins-on-calcium-influx-and-peptidyl-arginine-deiminase-pad-expression-in-macrophage/