Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Nintedanib, an intracellular inhibitor of tyrosine kinases, has shown promise in clinical trials by inhibiting key processes associated with the advancement of lung fibrosis. Its effectiveness has been demonstrated in treating idiopathic pulmonary fibrosis (IPF) as well as Systemic Sclerosis associated Interstitial Lung Disease (SSc-ILD). In this meta-analysis, we assessed the effectiveness of nintedanib in treating fibrosing interstitial lung diseases in both scleroderma and non-scleroderma patients.
Methods: We conducted a systematic review and meta-analysis of studies that investigated the effect of nintedanib on the annual decline of Forced Vital Capacity (FVC) in patients with fibrosing interstitial lung disease. We performed a comprehensive search in the databases of PubMed/MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception through May 2023. We included all randomized controlled trials. We excluded observational studies, abstracts, animal studies, case reports, reviews, editorials, and letters to editors. From each study, we collected the rate of annual reduction of FVC in both treatment and control groups. The treatment group included patients with fibrosing lung disease treated with nintedanib. The primary outcome was the rate of annual reduction in FVC measured in millimeters per year assessed after 52 weeks of follow-up. The random-effects model was used to calculate the mean differences (MD), and confidence intervals (CI). A p value < 0.05 was considered statistically significant. Heterogeneity was assessed using the Higgins I2 index.
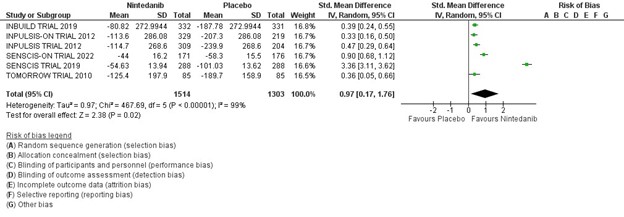
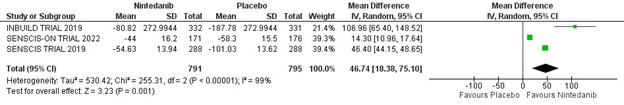
Results: Five randomized controlled trials involving 2817 patients were included in the meta-analysis. Patients were randomized to treatment and placebo groups in almost 1:1 ratio. The rate of annual decline in FVC was significantly lower in all patients with fibrosing lung disease treated with nintedanib (MD 0.97, 95% CI 0.17-1.76, p < 0.02. I2 = 99%). Three of the five randomized trials included 1586 patients with known scleroderma associated interstitial lung disease. The annual reduction in the FVC was also significantly lower in scleroderma patients treated with nintedanib (MD 46.74, 95% CI 18.38-75.10, p < 0.001, I2 = 99%).
Conclusion: Our meta-analysis revealed that patients treated with nintedanib experienced a decelerated rate of interstitial lung disease progression compared to those who received a placebo, regardless of the underlying cause of the fibrosing interstitial lung disease.
To cite this abstract in AMA style:
Khader Y, Rawish F, Abughrbyeh A, Davis S, Sidiki S, Safavi P, Altorok N. The Effectiveness of Nintedanib in Treating Fibrosing Interstitial Lung Disease in Both Scleroderma and Non-scleroderma Patients: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-effectiveness-of-nintedanib-in-treating-fibrosing-interstitial-lung-disease-in-both-scleroderma-and-non-scleroderma-patients-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effectiveness-of-nintedanib-in-treating-fibrosing-interstitial-lung-disease-in-both-scleroderma-and-non-scleroderma-patients-a-systematic-review-and-meta-analysis/