Session Information
Date: Friday, November 6, 2020
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose:
Avacopan, a novel, orally-administered selective antagonist of C5aR, was recently evaluated in a pivotal Phase 3 randomized clinical trial where its use in patients with ANCA-associated vasculitis, also receiving cyclophosphamide/azathioprine or rituximab, demonstrated superiority compared with the prednisone group. Eighty-one percent of subjects had renal vasculitis, with renal function being a major outcome predictor. This analysis focuses on changes observed in renal function during the trial.
Methods: Subjects were randomized 1:1 to receive either standard prednisone therapy or avacopan 30 mg twice daily. Both treatment groups received either cyclophosphamide followed by azathioprine, or rituximab. The primary efficacy endpoints were the proportion of subjects achieving disease remission at Week 26, and sustained disease remission at Week 52 as measured by the Birmingham Vasculitis Activity Score (BVAS). Renal function was assessed based on the estimated glomerular filtration rate (eGFR), and albuminuria, based on the albumin:creatinine ratio.
Results: 330 subjects were dosed: 164 in the prednisone group, 166 in the avacopan group. Primary endpoints, remission Week 26: avacopan 72.3% vs. prednisone 70.1% (P< 0.0001 non inferiority); avacopan 65.7% vs. prednisone 54.9% at Week 52 (avacopan statistically superior P=0.0066). Time to relapse from time of remission (BVAS=0) was longer for the avacopan group (P=0.0091 Log-rank test of difference). The hazard ratio of time to relapse for avacopan:prednisone was 0.46, (95% confidence interval: 0.25, 0.84), Figure 1. Efficacy was observed across newly-diagnosed vs. relapsed disease, PR3- vs. MPO-ANCA, granulomatosis with polyangiitis vs. microscopic polyangiitis, cyclophosphamide vs. rituximab, and men vs. women.
In subjects with renal disease at baseline, the avacopan group had a greater increase in eGFR vs. the prednisone group (7.3 mL/min/1.73 m2 vs. 4.1 mL/min/1.73 m2) (P=0.029). The difference was greatest in subjects with a baseline eGFR < 30 mL/min/1.73 m2 (Figure 2). There was a more rapid reduction in albuminuria in the avacopan group (-40%) compared to no change in the prednisone group at 4 weeks; the overall reduction at Week 52 was similar between groups.
The avacopan group had a favorable safety profile compared to the prednisone group. There were 166 serious adverse events in the prednisone group compared to 116 in the avacopan group, and 31 serious infections vs. 25 in the avacopan group. SAEs of WBC count decreases occurred in 4.9% of subjects in the prednisone group vs. 2.5% in the avacopan group, and liver function test increases in 3.7% vs. 5.4%, respectively.
Conclusion: Avacopan offers a new treatment option in ANCA-associated vasculitis. In addition to an improved sustained remission outcome, the avacopan group had greater improvement in renal function compared to standard prednisone therapy. These findings suggest the potential for better long-term outcomes with avacopan for patients with renal disease than current standard of care treatment and provide intriguing insights into subclinical renal disease activity in ANCA-associated vasculitis.
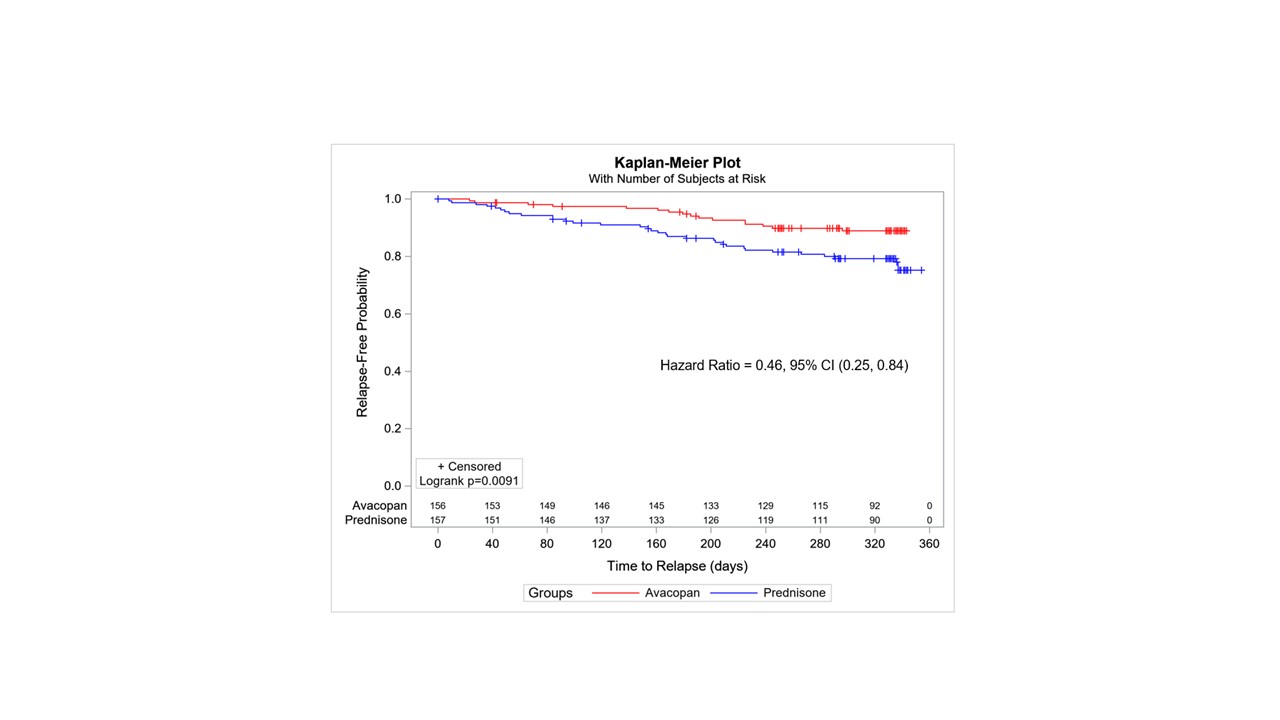 Figure 1: Time to Relapse in the ADVOCATE Study
Figure 1: Time to Relapse in the ADVOCATE Study
 Change in Estimated Glomerular Filtration Rate (eGFR) in Subjects with eGFR < 30mL/min/1.73 m2 at Baseline
Change in Estimated Glomerular Filtration Rate (eGFR) in Subjects with eGFR < 30mL/min/1.73 m2 at Baseline
To cite this abstract in AMA style:
Merkel P, Bekker P, Yue H, Kelleher C, Schall T, Jayne D. The Effect on Renal Function of the Complement C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-effect-on-renal-function-of-the-complement-c5a-receptor-inhibitor-avacopan-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-on-renal-function-of-the-complement-c5a-receptor-inhibitor-avacopan-in-anca-associated-vasculitis/
