Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Ear, nose and throat (ENT) manifestations are common in ANCA associated vasculitis (AAV), particularly the PR3 (proteinase 3) sub type. There is unmet need for drugs to target these manifestations. Granuloma formation is characteristic of PR3 AAV. Development of PR3-promoted monocyte-derived granuloma using cells from patients with PR3 AAV is inhibited by IL6 blockade while in a zebrafish model PR3 induced granuloma formation was inhibited by niclosamide, an IL6 STAT3 pathway inhibitor1. We therefore hypothesised that, in patients with AAV and ENT symptoms, intranasal niclosamide could reduce nasal vasculitic symptoms whilst on therapy.
Methods: The PROTECT-V trial was a randomised, double blind, placebo controlled platform trial evaluating pre-exposure prophylaxis agents against COVID19 infection2. This post hoc analysis includes a subset of patients recruited from a single centre, with AAV, enrolled into the intranasal niclosamide arm of the trial. Intranasal niclosamide or matched placebo was administered twice daily for up to 9 months. Patients were excluded from this analysis if they received treatment for less than 2 weeks. Using electronic patient records, we extracted clinical data from 9 months before, during trial drug administration and 9 months after the trial period and categorised participants into those with active and inactive vasculitic nasal disease. Both participants and researchers were blinded during this post hoc analysis.
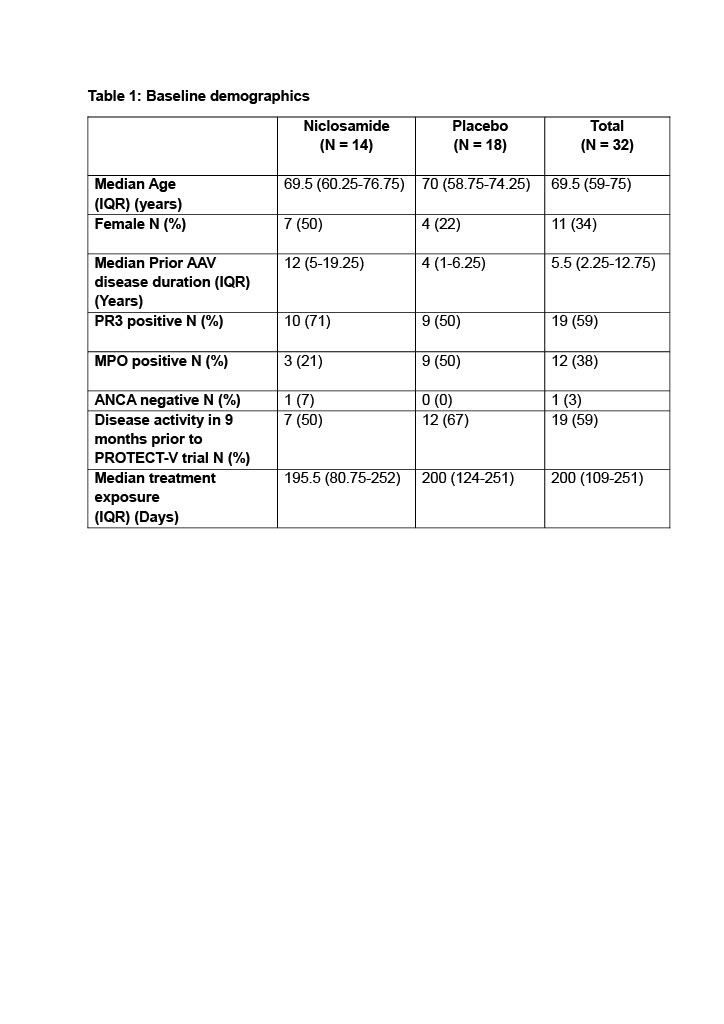
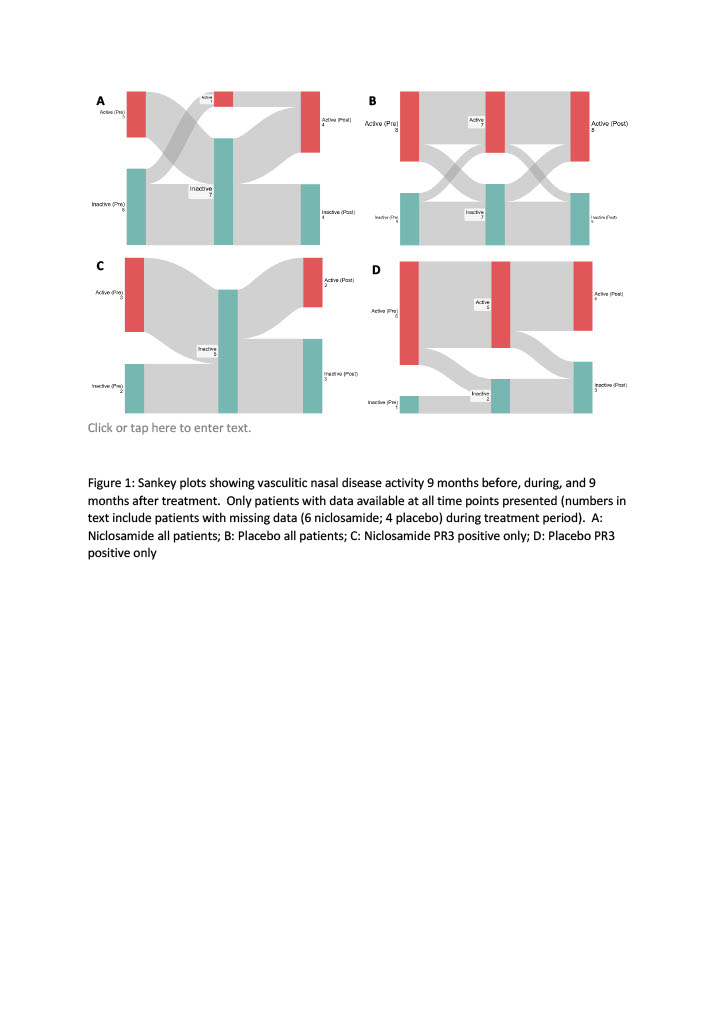
Results: 32 (14 niclosamide; 18 placebo) patients with AAV are reported. Overall, median age was 70 years (IQR 59-75); 11 (34%) were female. Median prior AAV disease duration was 5.5 years (IQR 2.3-12.8), and 19 (59%) had active disease in the 9 months prior to enrolment into the trial. Median trial treatment exposure was 200 days (IQR 109-251); 189 (IQR 81-252) for the niclosamide and 200 (124-251) for the placebo group (Table 1). The proportion of patients with active nasal disease remained unchanged in the placebo group (44% before trial treatment; 39% during and 44% after). However, in the niclosamide group, a smaller proportion of patients had active nasal disease whilst receiving drug (29% before, 7% during and 43% after) (p=0.0396 Chi-squared analysis during treatment) (Figure 1). The effect was more pronounced when restricted to PR3 patients, with 0/10 (0%) in the niclosamide and 5/9 (56%) in the placebo group reporting nasal disease activity whilst on drug.
Conclusion: Niclosamide has biological plausibility as a potential treatment for AAV. The PROTECT-V trial provided a unique opportunity to investigate this hypothesis using placebo controlled blinded data. This post hoc analysis is limited by small sample size and retrospective data collection for vasculitis activity. However, these data lend support for a role of the IL6 STAT3 pathway in nasal AAV, and intranasal niclosamide or other drug candidates in this pathway warrant further evaluation.
References
1 Henderson S et al. PR3 promotes formation of multinucleated giant cells and granuloma-like structures in patients with GPA. Ann Rheum Dis. 2023 Jun 82(6) 848-856
2 Humphrey T et al. PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V) 2023 Trials 24 185
To cite this abstract in AMA style:
Lim B, Qian W, Dowling F, Chen-Xu M, Salama A, Smith R. The Effect of Intranasal Niclosamide on Nasal Symptoms in Patients with ENT Manifestations of ANCA-Associated Vasculitis (AAV): Post Hoc Analysis of Subset of Patients Recruited to the PROTECT-V Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-intranasal-niclosamide-on-nasal-symptoms-in-patients-with-ent-manifestations-of-anca-associated-vasculitis-aav-post-hoc-analysis-of-subset-of-patients-recruited-to-the-protect-v-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-intranasal-niclosamide-on-nasal-symptoms-in-patients-with-ent-manifestations-of-anca-associated-vasculitis-aav-post-hoc-analysis-of-subset-of-patients-recruited-to-the-protect-v-trial/