Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Fostamatinib was developed for the treatment of RA and improved signs and symptoms of RA 1, but did not improve modified Total Sharp Score (mTSS) in the phase III OSKIRA-1 study. Previous publications have shown that interventions with tocilizumab (anti-IL6 receptor antibody) can modulate bone balance, and both cartilage and synovial turnover in a dose-dependent manner 2,3,4,5. To better understand the underlying mechanism of fostamatinib changes in biomarkers of joint tissue turnover were prospectively evaluated in response to fostamatinib treatment in patients with rheumatoid arthritis (RA) from the Phase III OSKIRA-1 study.
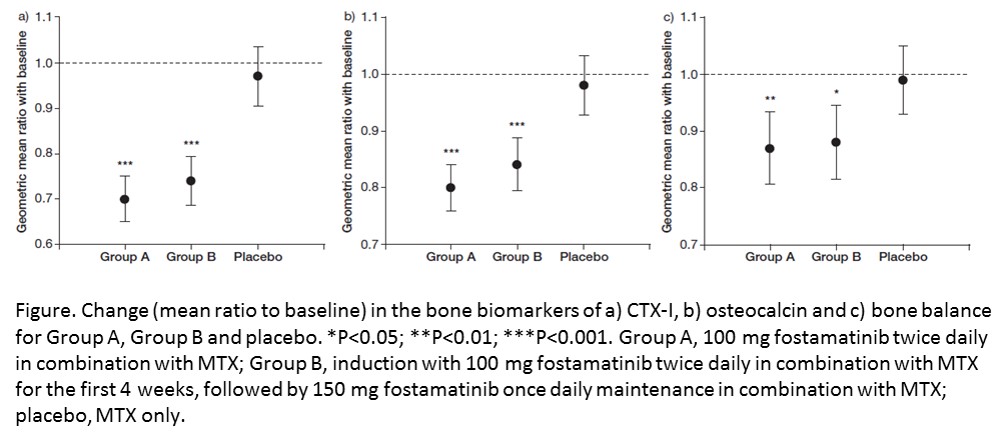
Methods: The biomarker evaluable population included 450 patients from the OSKIRA-1 trial. All patients received methotrexate and were randomly assigned to Group A (fostamatinib 100 mg bid), Group B (fostamatinib 100 mg bid for 4 weeks followed by fostamatinib 150 mg once daily) or placebo. Serum biomarkers of bone resorption (CTX‑I), bone formation (osteocalcin and type I pro-collagen N-terminal pro-peptide [PINP]), joint tissue degradation (C1M, C2M, C3M and matrix metalloproteinase [MMP]-3) and inflammation (MMP-mediated C-reactive protein [CRPM] and interleukin-6 [IL-6]) were assessed at baseline and Week 24. CTX-I/osteocalcin ratio was evaluated as a measure of bone balance 2.
Results: Fostamatinib significantly reduced a bone resorption biomarker at Week 24, with reductions of 23‒28% in CTX-I levels versus placebo (P<0.001), and statistically significantly improved biomarker bone balance versus placebo (P<0.05) (figure). However, no statistical significant changes for fostamatinib versus placebo were observed for C1M, a soft tissue marker shown to be predictive for structural efficacy 5, or for C2M, C3M and CRPM. Fostamatinib showed a significant decrease in MMP-3 levels (P=0.004) and IL-6 levels (P=0.004), although only in the high-dose group for IL-6.
Conclusion: Fostamatinib demonstrated a significant effect on the overall bone balance, but not on other biomarkers of joint tissue turnover, consistent with the structural data. Early and comprehensive analysis of biomarkers that encompass synovium, cartilage and bone metabolism may facilitate early clinical decision-making in RA drug development. 1Taylor PC et al. Ann Rheum Dis 2015. 2Karsdal MA et al. Semin Rheum 2012. References:3Bay-Jensen AC et al. Arthritis Res Ther 2016. 4Bay-Jensen AC et al Semin Arthritis Rheum 2014. 5Siebuhr A et al. Arthritis Res Ther 2013.
To cite this abstract in AMA style:
Platt A, Bay-Jensen AC, Braddock M, Jenkins M, Musa K, Thudium CS, Kjelgaard-Petersen CF, Graham E, Keeler S, Slynn G, Weinblatt M, Karsdal MA. The Effect of Fostamatinib with Methotrexate on Circulating Biomarkers of Synovium, Cartilage and Bone Metabolism: Potential Utility for Clinical Development Decision Making in Rheumatoid Arthriti [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-fostamatinib-with-methotrexate-on-circulating-biomarkers-of-synovium-cartilage-and-bone-metabolism-potential-utility-for-clinical-development-decision-making-in-rheumatoid-arthriti/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-fostamatinib-with-methotrexate-on-circulating-biomarkers-of-synovium-cartilage-and-bone-metabolism-potential-utility-for-clinical-development-decision-making-in-rheumatoid-arthriti/