Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpA) are commonly diagnosed in young males in their reproductive years. However, only a few studies have investigated the influence of biologic treatment on semen analysis in male patients with spondyloarthritis (SpA).
Aim: We aimed to evaluate the effects of biologic treatments for PsA and AxSpA on sperm parameters compared to those before treatment and to healthy controls (HC).
Methods: Consecutive PsA and AxSpA patients (age range 18-50 years), who fulfilled the classification criteria for PsA (CASPAR) and AxSpA (ASAS), and were planned to initiate a biologic therapy, were recruited prospectively. HC were recruited from candidate sperm donors at the male fertility clinic.
On the first visit, each patient underwent a comprehensive clinical assessment that included history taking, physical examination, measurement of disease activity scores (MDA, DAPSA, CPDAI & PASDAS for PsA and ASDAS for AxSpA) and questionnaires of quality of life. Sperm collection and analysis were performed on the day of clinical assessment. Sperm analysis was performed according to the World Health Organization 2010 guidelines. In addition, sperm DNA-fragmentation test was performed.
After mean of 173 days (± 71.5) of biologic treatment each patient was evaluated on a second visit, that included the same clinical evaluation and repeated sperm analysis.
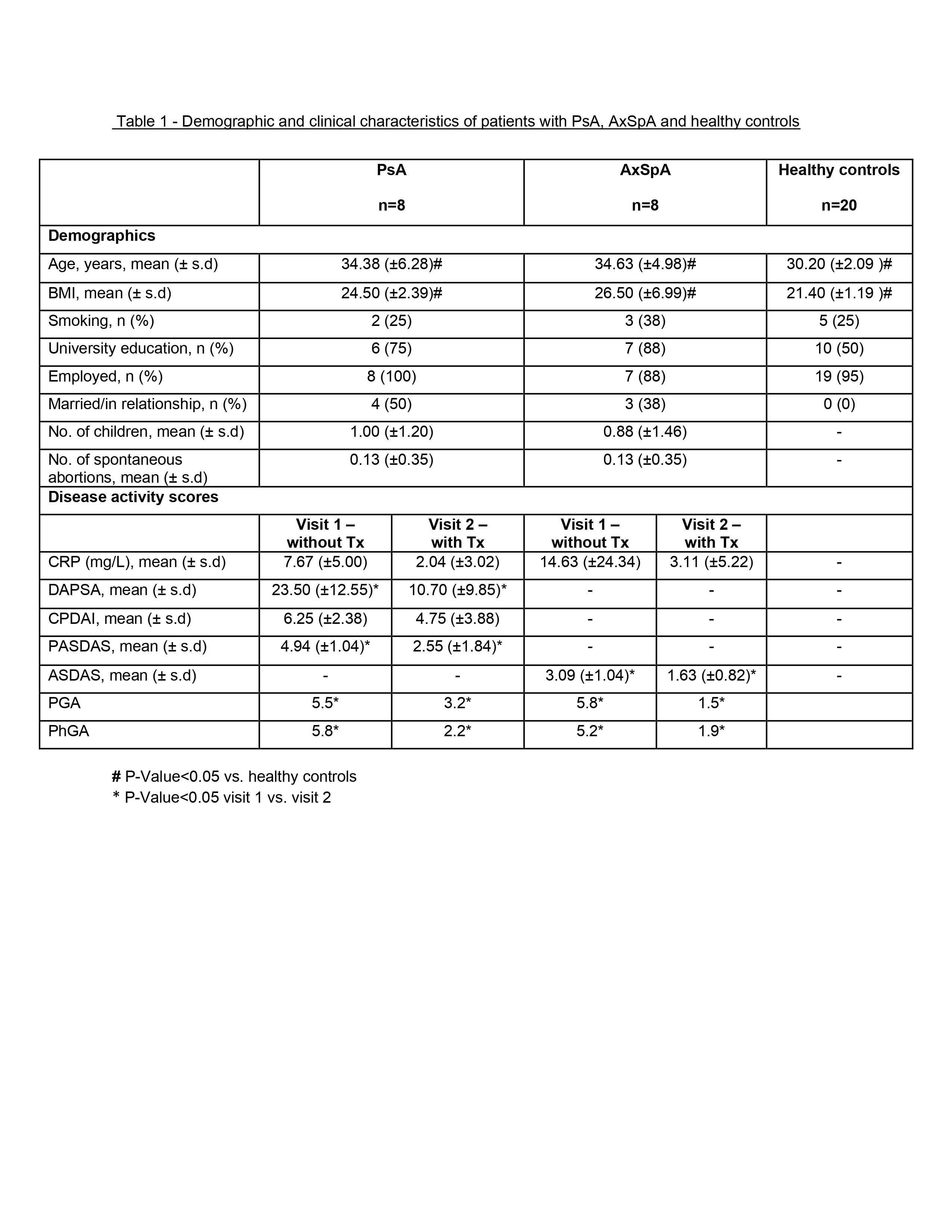
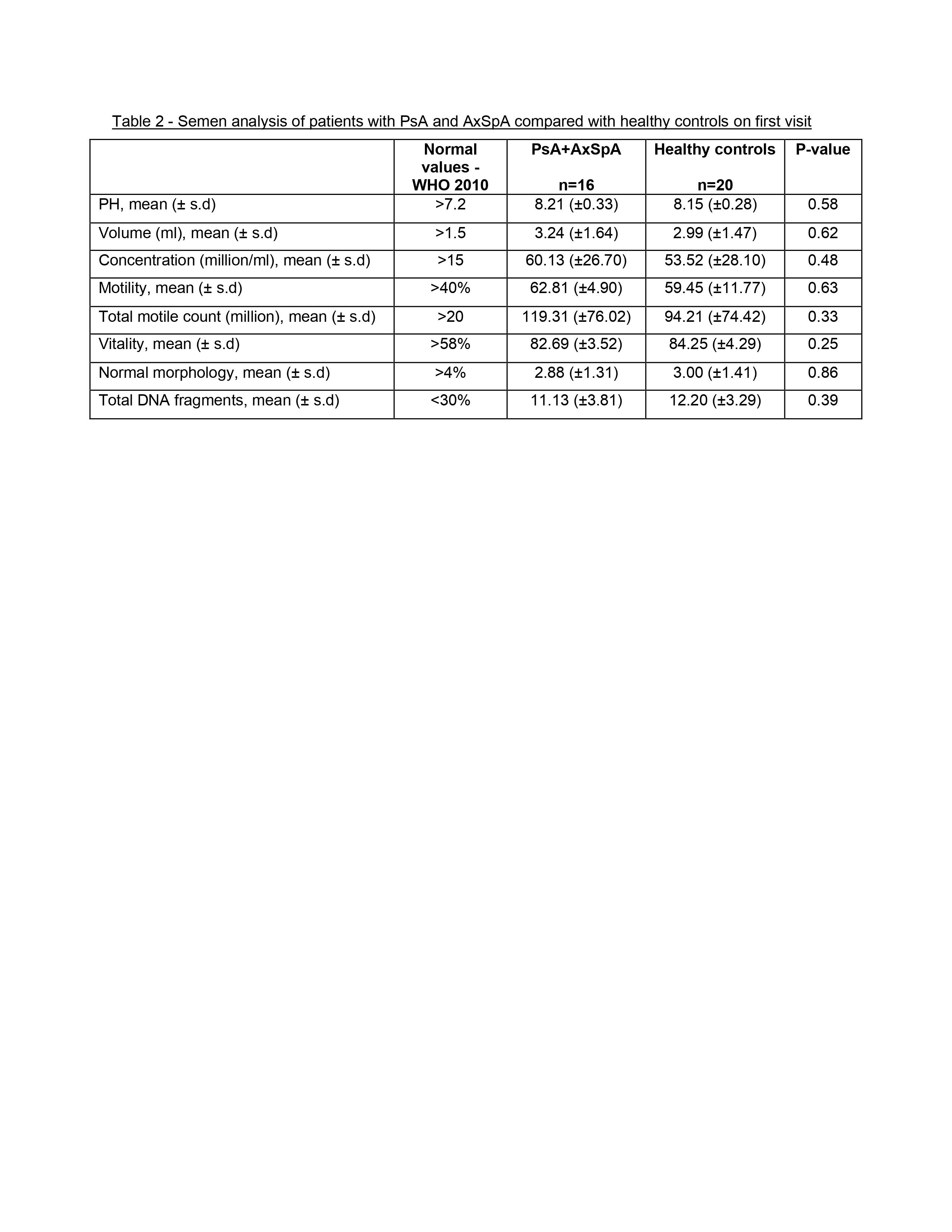
Results: 16 patients (8 PsA and 8 AxSpA) and 20 HC were included. Demographics and clinical characteristics are presented in Table 1. On the first visit (before initiation of biologics), semen parameters analysis, including morphology, concentration, motility, vitality and DNA fragmentation, of PsA and AxSpA patients were similar to those of HC (p >0.05) (Table 2).
Following sperm collection, 14 patients started treatment with TNF inhibitors and 2 patients started treatment with Secukinumab (anti-IL17). On the second visit, disease activity scores and CRP levels improved (most of them significantly, p< 0.05) compared to the initial evaluation (Table 1).
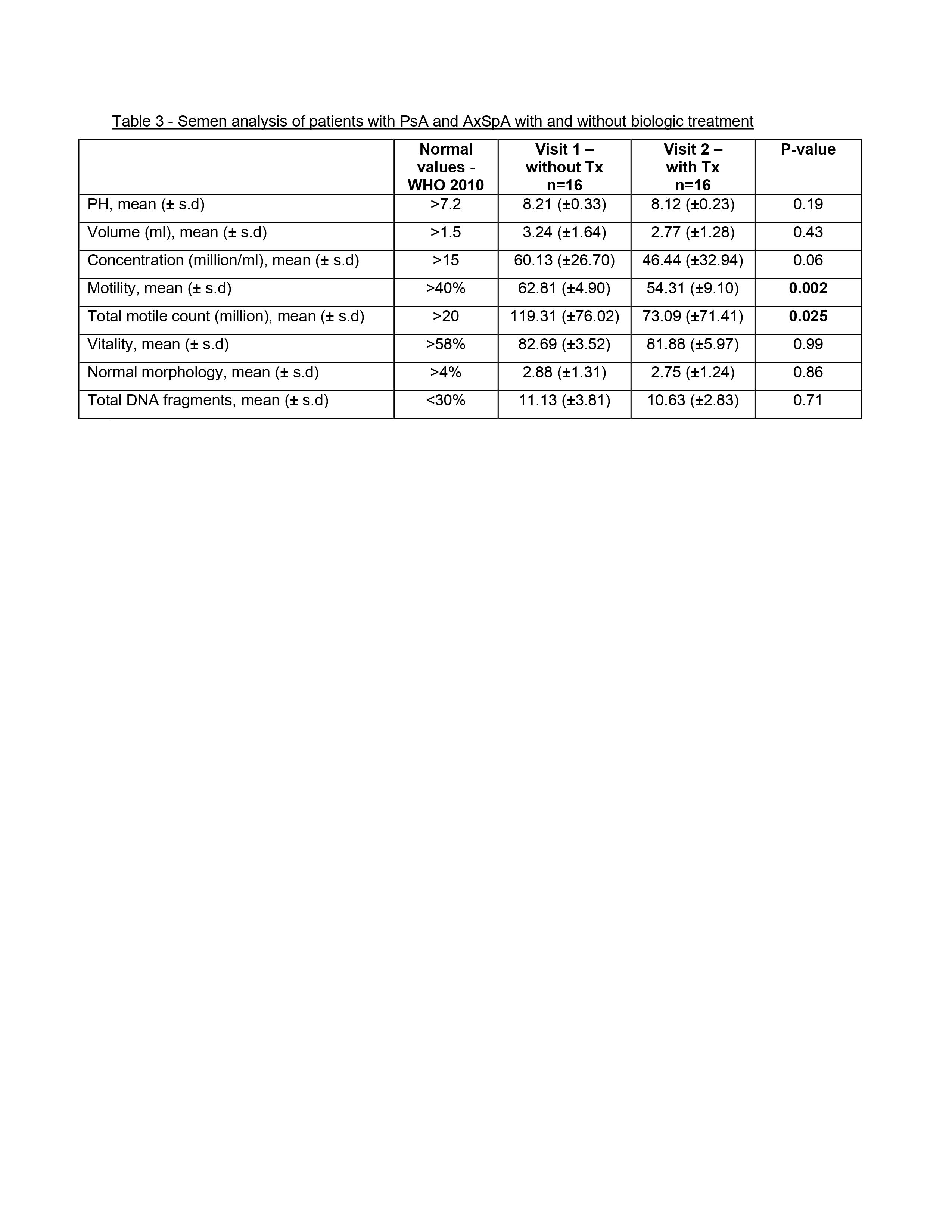
A comparison of the semen parameters before versus with biologic therapy, demonstrated that most parameters including morphology, concentration, vitality and DNA fragmentation were similar. However, motility was lower with biologic therapy compared to values before initiating treatment (54.31 (±9.10) vs. 62.81 (±4.90), respectively p< 0.01) (Table 3). Nevertheless, motility values for both groups were within the normal range ( >40%).
Conclusion: In this prospective longitudinal study evaluating semen analysis in spondyloarthropathies, the semen quality of PsA and AxSpA patients was comparable to HC in all parameters before treatment initiation. Biologic drugs (TNF inhibitors and anti-IL17) did not have a significant effect on sperm analysis. Therefore, men do not need cryopreservation of semen before treatment initiation nor a drug free interval prior to conception.
To cite this abstract in AMA style:
Meridor K, Barda S, Pel s, Nevo s, Berman J, paran d, Elkayam O, Polachek A. The Effect of Biologic Treatment for Psoriatic Arthritis and Axial Spondyloarthritis on Semen Parameters – a Longitudinal Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-biologic-treatment-for-psoriatic-arthritis-and-axial-spondyloarthritis-on-semen-parameters-a-longitudinal-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-biologic-treatment-for-psoriatic-arthritis-and-axial-spondyloarthritis-on-semen-parameters-a-longitudinal-study/