Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Enthesitis is a key lesion in psoriatic arthritis (PsA). The Diagnostic Ultrasound Enthesitis Tool (DUET) study aimed to develop and validate a novel sonographic scoring system for enthesitis to aid PsA diagnosis.
Methods: We prospectively enrolled patients with early PsA, psoriasis alone, and people with non-inflammatory musculoskeletal symptoms as controls, across 17 centers. Participants underwent ultrasound assessment of 16 entheseal sites in the upper and lower extremities, performed by local sonographers. Images were centrally reviewed by 3 readers to derive a consensus score for inflammatory lesions (hypoechogenicity [0–1], thickening [0–1], power Doppler [PD, 0–3]) and structural lesions (calcification [0–3], enthesophyte [0–3], erosion [0–1]). Using a stepwise approach with logistic regression models stratified by age, we identified optimal combinations of lesions and their weighting, and entheseal sites that maximized the ability to distinguish PsA from controls. Data from the discovery cohort, supplemented by an existing validation dataset, were used to determine cut-off points that optimize sensitivity while maintaining specificity above 70%. We further evaluated the association between the DUET score and PsA disease activity measures.
Results: We analyzed 213 patients with PsA (mean duration 1.9±3.4 yrs), 100 with psoriasis and 106 controls. The mean age was 49.9±14.6 and 53.7% were females. Tenderness at ≥1 entheseal site was observed in 70% of PsA patients, 49% of controls and 33% of psoriasis (SPARCC score: PsA: 3.2, control: 2.5, psoriasis: 1.2). Initial evaluation of 4 inflammatory and 5 structural sub-scores across 16 entheseal sites yielded 48 candidate scores. Stratification by age improved discriminative performance. The best performing score (DUET score), comprising inflammatory (hypoechogenicity + thickening + 2×PD) and structural (enthesophyte + 3×erosion, Fig. 1) sub-scores at the Achilles, patellar tendon (patellar insertion), and triceps tendon, achieved an overall AUC of 0.65. Age-specific cut-offs (age cut off 55) yielded specificity of 73–74% and sensitivity of 47–49% (Fig. 2). In the validation cohort (101 PsA, 69 controls), specificity improved to 74–100% with comparable sensitivity (49–52%). Sensitivity increased up to 63% in patients with tender entheseal sites (Fig. 3). The mean DUET score was significantly higher in PsA compared with controls and psoriasis (9.07 vs. 5.48 and 6.38, respectively; p< 0.01 for both, Fig. 3). Scores were elevated among obese patients, those with tender entheses, and those about to escalate PsA therapy.
Conclusion: DUET is a newly developed sonographic tool that may assist in the diagnosis of PsA, particularly among individuals with tender entheseal sites. The findings suggests that large joint enthesitis is not a universal feature of early PsA.
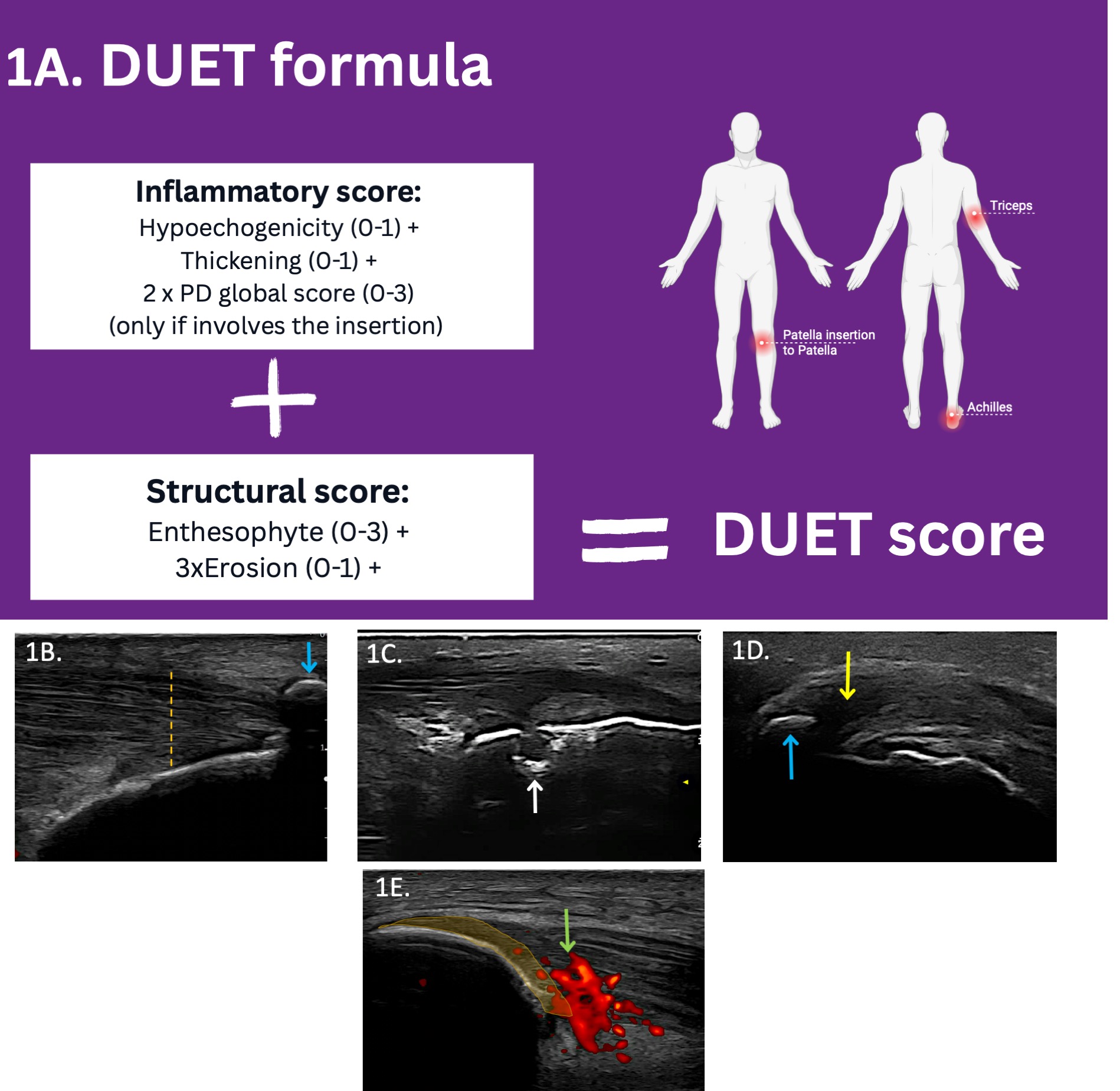 Figure 1: A summary of the DUET score. 1A. The DUET score formula is calculated based on a total of 6 entheseal sites: the Achilles and triceps insertions, and the patellar insertion to the patella. 1B–E. Depiction of selected elementary lesions and their corresponding DUET scores:
Figure 1: A summary of the DUET score. 1A. The DUET score formula is calculated based on a total of 6 entheseal sites: the Achilles and triceps insertions, and the patellar insertion to the patella. 1B–E. Depiction of selected elementary lesions and their corresponding DUET scores:
1B. Achilles tendon insertion showing thickening (1 point, vertical orange line) and enthesophyte (3 points, blue arrow); 1C. Achilles tendon insertion with erosion (1 point × 3, white arrow); 1D. Triceps tendon insertion with hypoechogenicity (1 point, yellow arrow) and enthesophyte (3 points, blue arrow); 1E. Patellar ligament insertion to the patella showing Doppler signal. The Doppler signal involves the area close to the bone (depicted by the orange marking), but the grading also considers Doppler intensity beyond this marking (3 points × 2).
.jpg) Figure 2: Performance of the DUET score.
Figure 2: Performance of the DUET score.
2A. Correlation of the DUET score with age across disease groups; 2B. Discriminative ability of the DUET score (by AUC) across various age categories; 2C–2D. Sensitivity, specificity, AUC, and distribution of DUET scores in PsA and controls for the selected cut-off points among individuals aged ≤55 years and >55 years in the discovery (Fig. 2C) and validation (Fig. 2D) cohorts.
.jpg) Figure 3: Correlation between the DUET score and clinical measures. 3A. Mean DUET score (by inflammatory and structural sub-scores) is higher in PsA compared to controls and psoriasis; 3B. DUET sensitivity by the number of tender entheses; 3C. DUET sub-score by PsA sub-groups.
Figure 3: Correlation between the DUET score and clinical measures. 3A. Mean DUET score (by inflammatory and structural sub-scores) is higher in PsA compared to controls and psoriasis; 3B. DUET sensitivity by the number of tender entheses; 3C. DUET sub-score by PsA sub-groups.
To cite this abstract in AMA style:
Eder L, Acayaba de Toledo R, Bakewell C, Carron P, Elnady B, Haddad A, Kavanaugh A, Afgani F, Katz A, Kohler M, marin j, Nguyen N, NZEUSSEU TOUKAP A, Polachek A, Rodriguez E, Rosemffet M, Singh A, Stoenoiu M, Szentpetery A, Tinazzi I, Yinh J, Yang M, Cook R, Kaeley G, Aydin S. The development and validation of a Diagnostic Ultrasound Enthesitis Score (DUET) for Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-development-and-validation-of-a-diagnostic-ultrasound-enthesitis-score-duet-for-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-development-and-validation-of-a-diagnostic-ultrasound-enthesitis-score-duet-for-psoriatic-arthritis/
