Session Information
Date: Sunday, November 5, 2017
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud's – Clinical Aspects and Therapeutics I
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: While prior observational studies have identified predictors of mortality in systemic sclerosis-interstitial lung disease (SSc-ILD), no studies have evaluated predictors of long-term mortality in a clinical trial, in which all patients receive standard care. The objective of this study was to identify predictors of mortality in patients who participated in the Scleroderma Lung Study (SLS) I1 and II2.
Methods: SLS I randomized 158 SSc-ILD patients to 1 year of oral cyclophosphamide (CYC) versus placebo. SLS II randomized 142 patients to 1 year of oral CYC followed by 1 year of placebo versus 2 years of mycophenolate (MMF). The FVC%-predicted and DLCO%-predicted were measured every 3 months for 2 years in both trials. 12 years after SLS I commenced, each study center contacted enrolled patients/designated surrogates to assess morbidity and mortality outcomes. Counting process cox proportional hazard modeling identified variables associated with mortality. A joint model of longitudinal FVC and survival data was used to internally validate the model. We externally validated the model using long-term mortality data from SLS II (up to 5 years of follow-up).
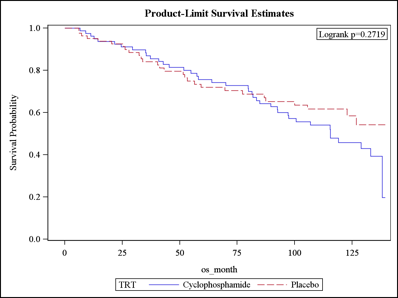
Results: Among SLS I patients, 43% died during the follow-up period (median follow-up: 8 years), and only 24% remained alive without organ failure. Where known, the cause of death was attributable most often to SSc. The most common type of organ failure was respiratory failure (N=31 of 33 organ failures) defined as the need for supplemental oxygen therapy (N=29) and/or lung transplantation (N=3). There was no significant difference in the time to death between patients randomized to CYC versus placebo (Figure 1). The Cox model identified the following mortality predictor variables: baseline skin score (HR 1.03; P=0.004), age (HR 1.06; P<0.0001), and the course of the FVC from baseline to 24 months (HR 0.98; P=0.022). The course of the FVC was a better predictor than the baseline FVC. The joint model identified the same variables associated with mortality. Using the SLS II data, the Cox model identified the same mortality predictor variables: baseline skin score (HR 2.08; P=0.021), age at randomization (HR 1.08; P=0.011), and the course of the FVC from baseline to 24 months (HR 0.79; P=0.020).
Conclusion: Treatment with 1-year of oral CYC for SSc-ILD did not significantly decrease long-term mortality compared with placebo. In addition to identifying traditional mortality risk factors in SSc (i.e. increased skin score and advanced age), this study found that a decline in FVC over 2 years was a better predictor of mortality than the baseline FVC. These findings suggest that early changes in surrogate measures of SSc-ILD progression may have important effects on long-term outcomes.
1Tashkin et al. NEJM 2006.
2Tashkin et al. Lancet Resp Med 2016.
Figure 1. Time to death in patients randomized to CYC (solid line) and placebo (dotted line).
To cite this abstract in AMA style:
Volkmann ER, Tashkin DP, Sim M, Khanna D, Roth M, Clements PJ, Furst DE, Keyes-Elstein L, Pinckney A, Goldmuntz E, Elashoff R, Sullivan K. The Course of the Forced Vital Capacity during Treatment for Systemic Sclerosis-Related Interstitial Lung Disease Predicts Long-Term Survival in 2 Independent Cohorts [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-course-of-the-forced-vital-capacity-during-treatment-for-systemic-sclerosis-related-interstitial-lung-disease-predicts-long-term-survival-in-2-independent-cohorts/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-course-of-the-forced-vital-capacity-during-treatment-for-systemic-sclerosis-related-interstitial-lung-disease-predicts-long-term-survival-in-2-independent-cohorts/