Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Previous data from the Corrona RA registry, conducted in a US clinical practice setting, demonstrated that patients (pts) with RA who were ACPA+ had a greater clinical response to abatacept than ACPA– pts, a relationship not observed with TNF inhibitors or tofacitinib.1,2 This comparative study evaluated the effectiveness of abatacept vs tofacitinib in pts with RA (ACR 1987 criteria) who were ACPA+.
Methods:
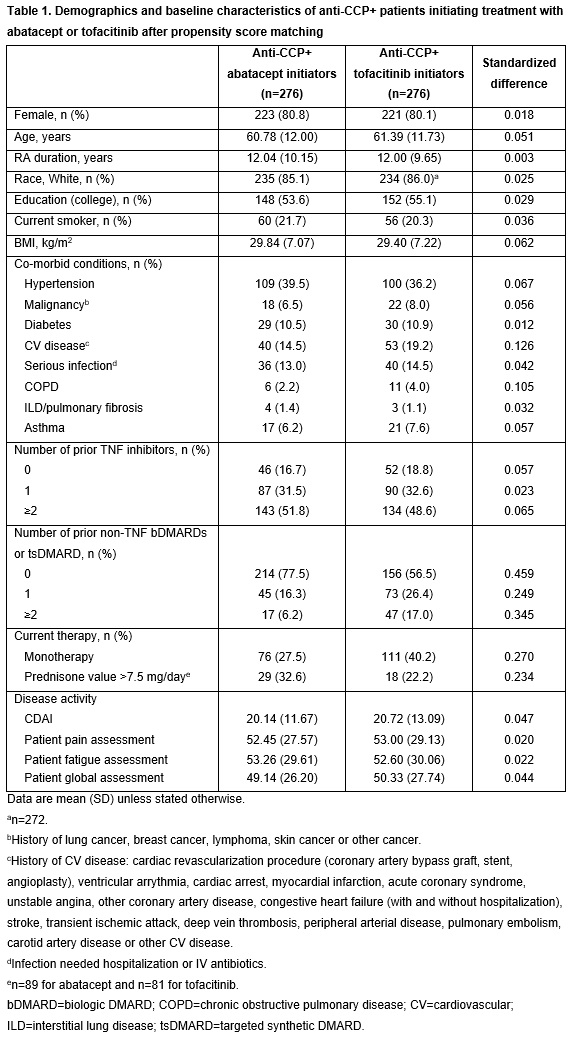
Pts from the Corrona RA registry who were aged ≥18 years and were anti-CCP+ (≥20 U/mL) before starting treatment, initiated abatacept or tofacitinib from Dec 2012 onwards, had 6-month follow-up data (including CDAI score at initiation and 6 months) and were not in remission at index date were included. Pts were frequency matched 1:1 based on the number of prior biologics (0, 1, ≥2) before 1:1 propensity score matching (PSM) based on: duration of RA, education, number of prior biologics, time on therapy, baseline CDAI, current smoker, history of malignancy and BMI. Primary (mean change in CDAI score) and secondary outcomes 6 months after index date were compared using mixed-effects models. Variables that remained unbalanced after PSM were used as covariates in adjusted multivariable models, including age, sex, race, work status, BMI, systolic blood pressure, history of non-TNF inhibitor biologic (b)DMARD use, history of cardiovascular (CV) disease, baseline CDAI and modified HAQ score. Standardized difference >0.1 indicates a meaningful difference between treatments.
Results:
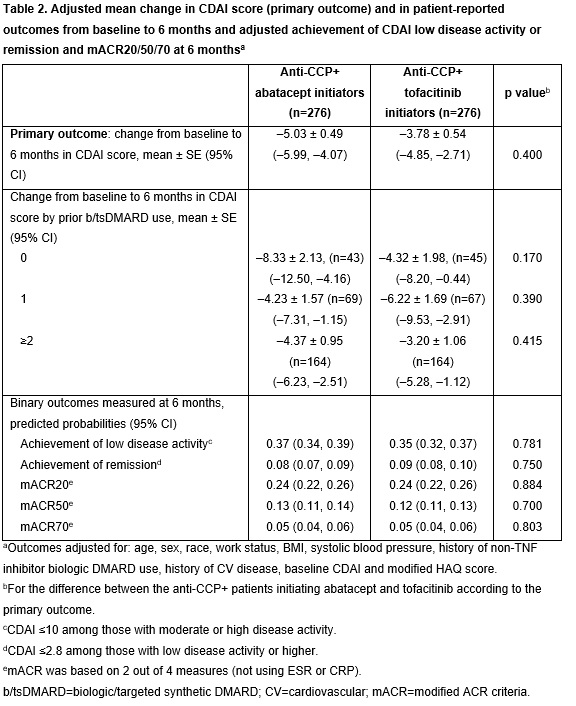
Following PSM, most baseline characteristics for the 276 pt pairs included in the abatacept and tofacitinib groups were well balanced with a standardized difference ≤0.1 (Table 1). Compared with those initiating tofacitinib, fewer pts initiating abatacept had received prior treatment with non-TNF bDMARDs, were on monotherapy or had CV disease or chronic obstructive pulmonary disorder, and more had a dose of prednisone >7.5 mg/day (Table 1). In the adjusted analyses, there was not a significant difference in mean change from baseline in CDAI score at 6 months in pts initiating treatment with abatacept vs tofacitinib (p=0.400; Table 2). Among pts stratified by prior b/targeted synthetic (ts)DMARD use, those who were b/tsDMARD-naïve at baseline had a numerically greater mean change in CDAI at 6 months in the abatacept vs tofacitinib group, although this difference was not statistically significant (p=0.170; Table 2). There were no significant differences between abatacept and tofacitinib for any of the secondary outcomes, although improvements in mean change in pain and fatigue at 6 months were numerically greater in pts receiving abatacept vs tofacitinib (Figure 1).
Conclusion: In adjusted analyses, ACPA+ pts with RA treated with abatacept vs those treated with tofacitinib did not have statistically significant differences in reduction of disease activity and pt-reported outcomes. Additional studies to explore these findings in a larger population would be of interest.
References:
- Harrold LR, et al. J Rheumatol 2018;45;32–39.
- Harrold LR, et al. Arthritis Rheumatol 2019;71(suppl 10):2415–2416 [abstract 1386].
Medical writing: Catriona McKay, PhD (Caudex)
To cite this abstract in AMA style:
Harrold L, Wittstock K, Kelly S, Han X, Shan Y, Moore P, Guo L, Khaychuk V. The Comparative Effectiveness of Abatacept versus Tofacitinib After 6 Months of Treatment in Patients with RA Who Were Anti-citrullinated Protein Antibody Positive at Baseline: Results from a US National Observational Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-comparative-effectiveness-of-abatacept-versus-tofacitinib-after-6-months-of-treatment-in-patients-with-ra-who-were-anti-citrullinated-protein-antibody-positive-at-baseline-results-from-a-us-natio/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-comparative-effectiveness-of-abatacept-versus-tofacitinib-after-6-months-of-treatment-in-patients-with-ra-who-were-anti-citrullinated-protein-antibody-positive-at-baseline-results-from-a-us-natio/