Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Gastrointestinal tract (GIT) symptoms are common amongst systemic sclerosis (SSc) patients.1 The Scleroderma Clinical Trials Consortium University of California Los Angeles Gastrointestinal Tract Questionnaire (GIT 2.0) allows a clinician to assess the preceding seven days of reflux, bloating/distention, diarrhea, constipation, soilage as well as the emotional and social impact of gastrointestinal symptoms into an absent-to- mild, moderate, or severe categorization, which can be trended over subsequent care visits.2,3 The Collaborative National Quality and Efficacy Registry (CONQUER) is an effort in the United States to collect data on state-of-the-art clinical practice at SSc centers of excellence in patients with < 5 years disease duration.4 In this study, we describe the SSc gastrointestinal tract natural history and association to medication use in CONQUER participants.
Methods: The inclusion criteria for this project included CONQUER participants that had completed a minimum of two serial GIT 2.0. Patients were categorized by total GIT 2.0 severity and subsequent categories were created: no change (none-to-mild), improvement in category, worsening in category, and no change (moderate-to-severe). Clinical features including demographics, disease duration, cutaneous subset, and autoantibodies as well as medication changes between care visits were examined in each of these categories. Medications were categorized as gastrointestinal tract targeted therapy, anti-fibrotic (nintedanib only), immunosuppression, or immunomodulatory drugs (hydroxychloroquine only). Analysis included a proportional odds model accounting for linear and mixed effects of described variables.
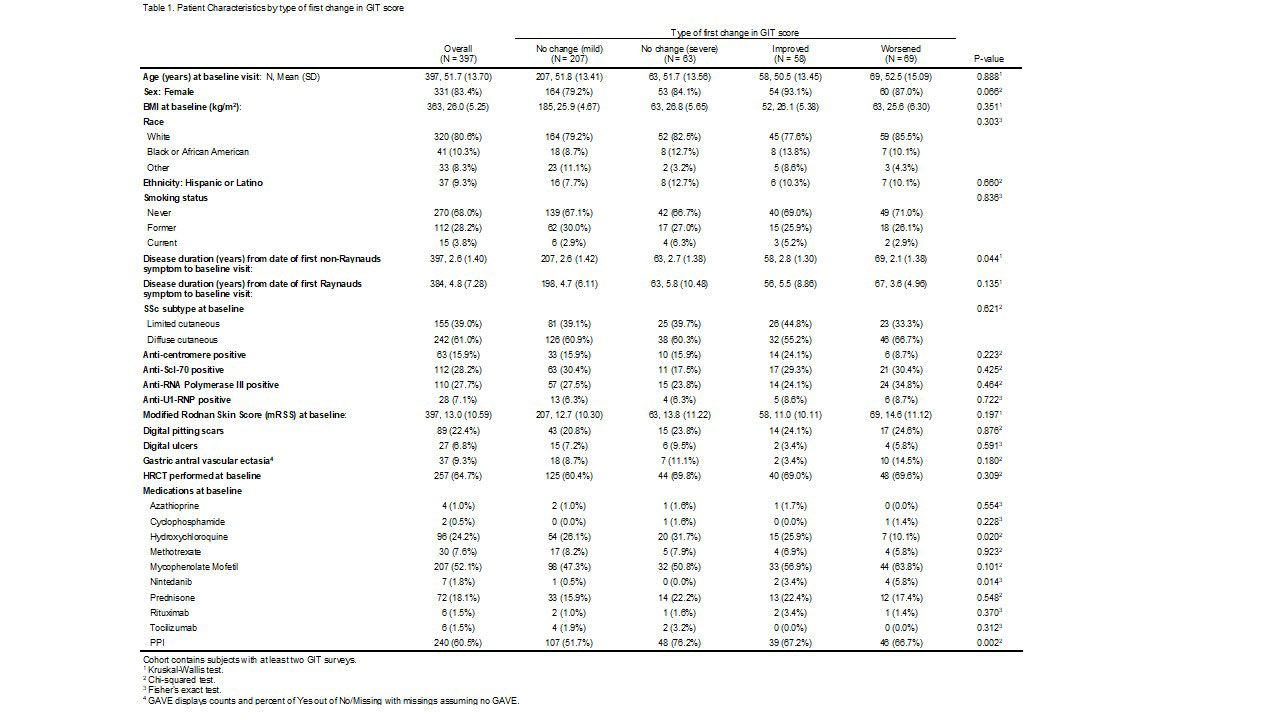
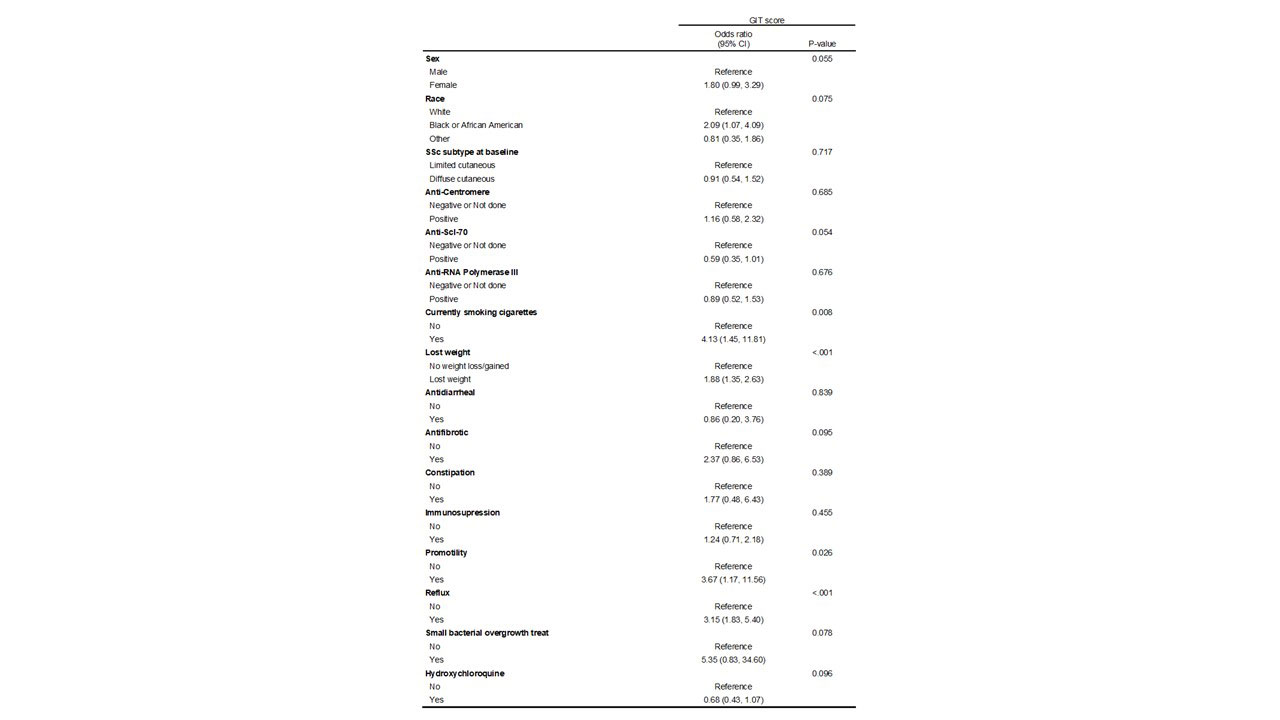
Results: At the time of data analysis on May 4, 2022, there were 399 enrolled CONQUER participants that met project inclusion criteria. The sociodemographic and disease characteristics of those 399 participants categorized by change in GIT symptoms are shown in Table 1. Most participants (n=208, 52%) had stable mild GIT symptoms at all observed clinical assessments. The timing of medication initiation in relationship to GIT survey administration is shown in Figure 1. On average, patients that had a change did so within the first year of enrollment in CONQUER. In all GIT categories, reflux medication and immunosuppression initiation preceded baseline visit whereas anti-fibrotic initiation occurred at or after baseline visit. There were no patients on IVIG or pirfenidone. The odds of having a worse GIT score at any visit is shown in Table 3 with significance found in weight loss, tobacco use, or the use of reflux or promotility medication.
Conclusion: This report from the CONQUER cohort, suggests that the majority of early patients have stable mild GIT disease. There is no clear association of immunosuppression or anti-fibrotic use with worsening GIT symptoms, but tobacco use is clearly associated, highlighting the importance of smoking cessation.
To cite this abstract in AMA style:
Luebker S, Frech T, Assassi S, Gordon J, Bernstein E, Steen V, Shah A, Hummers L, Richardson C, Khanna D, Castelino F, Chung L, Hant F, Shanmugam V, VanBuren J, Alvey J, Harding M, Evnin L, Sandorfi N. The Collaborative National Quality and Efficacy Registry for Scleroderma: Association of Medication Use on Gastrointestinal Tract Symptoms in Early Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-collaborative-national-quality-and-efficacy-registry-for-scleroderma-association-of-medication-use-on-gastrointestinal-tract-symptoms-in-early-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-collaborative-national-quality-and-efficacy-registry-for-scleroderma-association-of-medication-use-on-gastrointestinal-tract-symptoms-in-early-disease/