Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Some patients with rheumatoid arthritis (RA) need switching therapy between biologic agents (Bio) and Janus kinase inhibitors (JAKi) when efficacy of Bio or JAKi is not enough.
The aim of this study was to investigate the clinical efficacy of switching therapy between Bio and JAKi in the patients with RA.
Methods: We evaluated inflammatory markers and disease activities for 12 weeks (W) after switching in the patients with RA who received the switching therapy from Tumor Necrosis Factor inhibitors (TNFi) to JAKi (TNFi-JAKi group, N=61; mean age 64.6±12.9 years old), from Interleukin-6 inhibitors (IL-6i) to JAKi (IL-6i-JAKi group, N=33; mean age 64.9±14.7 years old), and from JAKi to IL-6i (JAKi-IL-6i group, N=8; mean age 65.5±14.7 years old).
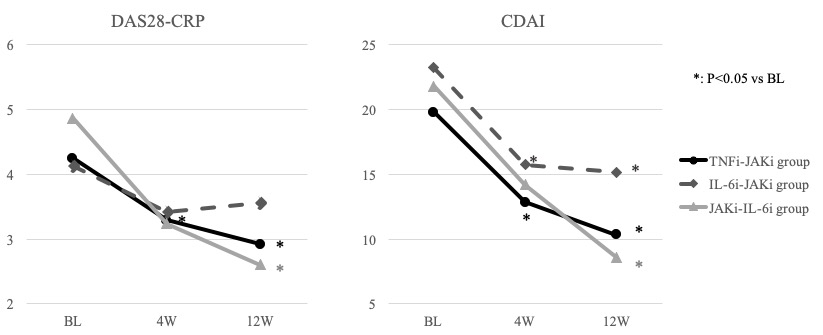
Results: The mean values of Matrix Metalloproteinase-3 (MMP-3) (ng/ml) of TNFi-JAKi, IL-6i-JAKi, and JAKi-IL-6i groups were 213.5±481.3, 237.5±179.4, and 268.5±138.4 at baseline (BL), and 118.9±126.3 (p=0.148), 183.6±192.7 (p=0.279), and 196.2±100.9 (p=0.254) after 4W (vs BL), and 104.0±96.1 (p=0.089), 176.6±120.8 (p=0.123), and 137.4±114.5 (p=0.077) after 12W (vs BL), respectively. The mean Disease Activity Score 28- C reactive protein (DAS28-CRP) of TNFi-JAKi, IL-6i-JAKi, and JAKi-IL-6i groups were 4.22±1.27, 4.13±1.39, and 4.87±1.35 at BL, and 3.28±1.37 (p< 0.001), 3.41±1.46 (p=0.805), and 3.24±1.85 (p=0.065) after 4W (vs BL), and 2.99±1.24 (p< 0.001), 3.56±1.45 (p=0.115), and 2.60±1.40 (p=0.005) after 12W (vs BL), respectively (Figure 1). The mean Clinical Disease Activity Index (CDAI) of TNFi-JAKi, IL-6i-JAKi, and JAKi-IL-6i groups were 19.8±11.8, 23.2±12.7, and 21.8±9.9 at BL, and 12.8±10.4 (p< 0.001), 15.7±12.5 (p=0.020), and 14.2±13.9 (p=0.228) after 4W (vs BL), and 10.3±9.8 (p< 0.001), 15.2±12.6 (p=0.012), and 8.6±9.5 (p=0.017) after 12W (vs BL), respectively (Figure 1). There were no significant differences in the mean values of MMP-3 for 12W after switching in all groups. The disease activities (both of DAS28-CRP and CDAI) significantly decreased after 4W in TNFi-JAKi group, and after 12W in TNFi-JAKi and JAKi-IL-6i groups (Figure 1). DAS28-CRP and CDAI kept to decrease for 12W after switching in TNFi-JAKi and JAKi-IL-6i groups, however, DAS28-CRP increased and CDAI did not decrease from 4W to 12W after switching in IL-6i-JAKi group (Figure 1).
Conclusion: There have not still been clear indications of the switching therapy between Bio and JAKi in the patients with RA. In this study, it may be considered that the switching therapy from TNFi to JAKi and from JAKi to IL-6i could be effective when RA patients treated with TNFi or JAKi need switching therapy due to insufficient clinical efficacy.
To cite this abstract in AMA style:
Choshi T, Mamoto K, Yamada Y, Okano T, Anno S, Orita K, Iida T, Tada M, Inui K, Koike T, Nakamura H. The Clinical Efficacy of Switching Therapy Between Biological Agents and JAK Inhibitors in the Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-clinical-efficacy-of-switching-therapy-between-biological-agents-and-jak-inhibitors-in-the-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-clinical-efficacy-of-switching-therapy-between-biological-agents-and-jak-inhibitors-in-the-patients-with-rheumatoid-arthritis/