Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Gumiganghwal-tang (GMGHT) is a traditional herbal medicine consisted of nine different herbs. GMGHT inhibit the production and mRNA expression of inflammatory cytokine production TNF IL B on LPS-stimulated peritoneal macrophages in a dose-dependent manner. It is empirically used for the treatment of inflammatory disease including common cold and various pain, but there are few reports on clinical trials to clarify its efficacy and safety. The current study aimed to investigate the clinical efficacy and safety of GMGHT in patients with knee OA.
Methods: This was a 6-week, multicenter, phase 2, double-blind, randomized, and placebo controlled study of GMGHT vs placebo. Eligible patients who fulfilled American College of Rheumatology criteria were randomized to receive either GMGHT or placebo. Clinical assessments included measurement of knee pain and function with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), patient global assessment (PGA), and knee pain scores every 2 weeks.
Results: A total of 128 patients enrolled (female n=117, 91.4%; mean age 58.7 ¡¾ 8.1 years) was enrolled. At baseline, pain VAS was 67.2¡¾1.4, 71.3¡¾1.6 (treatment and placebo group, respectively, p=0.84) and total WOMAC score was 55.2¡¾1.6, 55.6¡¾1.5 (p=0.84). At 6-week, pain VAS was 43.0¡¾2.5, 61.6¡¾2.5 (treatment and placebo group, respectively, p<0.01) and total WOMAC score was 34.1¡¾2.4, 46.9¡¾1.8 (p<0.01, Figure 1). None of patients discontinued because of treatment emergent adverse events. Expected adverse event including dyspepsia, liver function abnormality, and low extremity edema was comparable between both groups (Table1).
Conclusion: Treatment of GMGHT resulted in significant improvement in pain, function and global assessment, and it was generally safe and well-tolerated in patients with OA. 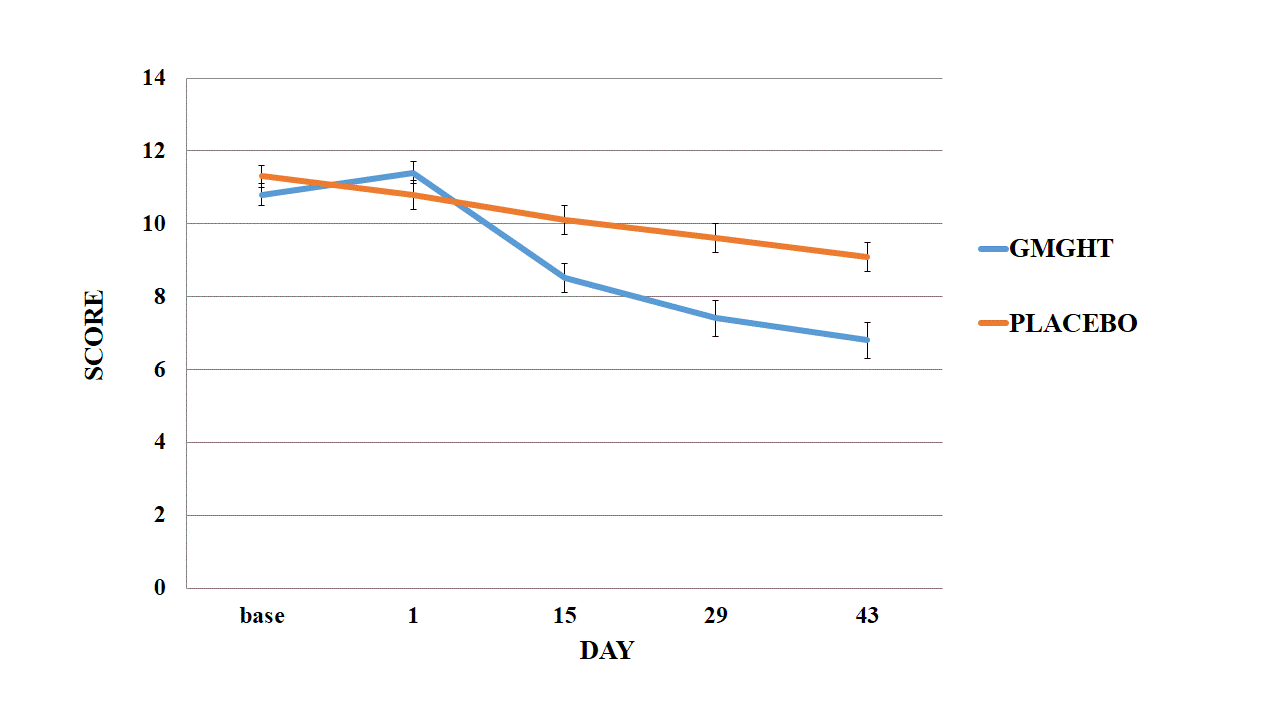 Figure 1. Outcome measures in patients with knee OA who were treated with GMGHT; change from baseline to week 6 in patient¡¯s pain VAS (A) and the total WOMAC score (B)
Figure 1. Outcome measures in patients with knee OA who were treated with GMGHT; change from baseline to week 6 in patient¡¯s pain VAS (A) and the total WOMAC score (B)
(A)
 |
(B) Table 1. Summary of adverse events
|
GMGHT (n=63) |
Placebo (n=67) |
p-value |
|
| Patient with any of adverse events, n (%) |
14(22.2) |
16(23.9) |
0.83 |
| Patient who discontinued due to with serious adverse events, n (%) |
1(1.6) |
1(1.5) |
1.00 |
| Gastrointestinal |
|
|
|
| Dysgeusia, n (%) |
3(4.8) |
4(6.0) |
1.00 |
| Dyspesia, n (%) |
2(3.2) |
4(6.0) |
0.68 |
| Constipation, n (%) |
0 |
1(1.5) |
– |
| Flatulance, n (%) |
2(3.2) |
3(4.5) |
1.00 |
| Lower extremity edema, n (%) |
1(1.6) |
1(1.5) |
1.00 |
| Laboratory abnormalitie |
|
|
|
| Transient LFT elevaion, n (%) |
2(3.2) |
1(1.5) |
0.61 |
| Transient Creatinine elevation, n (%) |
2(3.2) |
1(1.5) |
0.61 |
To cite this abstract in AMA style:
Chang SH, Kang MI, Nah SS. The Clinical Efficacy and Safety of the Gumiganhwal-Tang in Knee Osteoarthritis: A Phase II Randomized Double Blind Placebo Controlled Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-clinical-efficacy-and-safety-of-the-gumiganhwal-tang-in-knee-osteoarthritis-a-phase-ii-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-clinical-efficacy-and-safety-of-the-gumiganhwal-tang-in-knee-osteoarthritis-a-phase-ii-randomized-double-blind-placebo-controlled-study/
