Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis (LN) affects over 50% of SLE patients. Twenty percent of LN patients develop end-stage renal disease (ESRD) within 10 years. The 2021 CREDENCE trial and subsequent studies in the general population demonstrated that sodium-glucose co-transporter 2 inhibitors (SGLT2i) were shown to slow the progression of chronic kidney disease in type 2 diabetes patients. However, experience in SLE is limited. We investigated changes in eGFR and proteinuria in SLE patients following SGLT2i initiation.
Methods: We studied 109 SLE patients longitudinally (86% female; 32% White, 60% African-American, 9% Other, 32% with diabetes, 60% with eGFR < 60, and 58% with UPCR < 0.50 gm) who started taking SGLT2i and had at least one clinical visit within three years before and/or after treatment initiation. eGFR was calculated using the 2021 CKD-EPI creatinine equation, and proteinuria was assessed using the urine protein-creatinine ratio (UPCR). Longitudinal mixed effects regression models with random slopes and intercepts were used to estimate changes in eGFR and UPCR before and after SGLT2i initiation. Models included terms for immediate level change and slope change starting 30 days after SGLT2i initiation, assuming linear trends in eGFR or UPCR. Subgroup analyses were done for diabetes mellitus status and baseline kidney function (eGFR < 60 vs. ≥60 mL/min/1.73m²).
Results: In the cohort, the mean annual decline in eGFR before SGLT2i initiation was -2.0 (P=0.0002). The immediate change in eGFR post SGLT2i showed a decline of -1.4 (P=0.17). The eGFR slope post SGLT2i improved by 0.43 (P=0.63, Table 1). The initial decline followed by improvement in GFR slope were similar to the results of the CREDENCE trial, but did not reach statistical significance. The improvement in eGFR slope was greater in those with diabetes and in those with eGFR < 60 (Table 2). This same benefit was shown in those with UPCR < 0.50g at SGLT2i initiation (Table 2, p=0.036) and also in those with UPCR < 0.30g at SGLT2i initiation (p=0.043).Next we looked at the effect of SGLT2i on UPCR. There was a significant reduction in the UPCR after starting SGLT2i in all SLE patients (Table 1). Contrary to the eGFR effects, the benefit was statistically significant in those without diabetes (Table 2, p < 0.001) and with baseline eGFR >60 (Table 2, p < 0.001).
Conclusion: In this analysis, SGLT2i use did not significantly reduce eGFR decline (although the results were in the correct direction), but had a significant improvement on proteinuria. This differs from the 2022 Phase I/II trial in SLE by Wang et al, which found the opposite to be true. Our subgroup effects are fascinating and go in opposite directions. The benefit of SGLT2i on GFR slope is seen in those WITH and GFR < 60 (neither were significant). In particular, protection of GFR slope was seen in those with LOW levels of UPCR, such as < 0.50 g and < 0.30 g. The benefit of SGLT2i on proteinuria is seen in the all patient analysis (p=0.0001) and in those without diabetes (p < 0.001) and eGFR > 60 (p < 0.0001). Both GFR protection and reduction in proteinuria are desirable goals in SLE, but our study found that GFR protection was not statistically significant in the all patient analysis, whereas proteinuria reduction was.
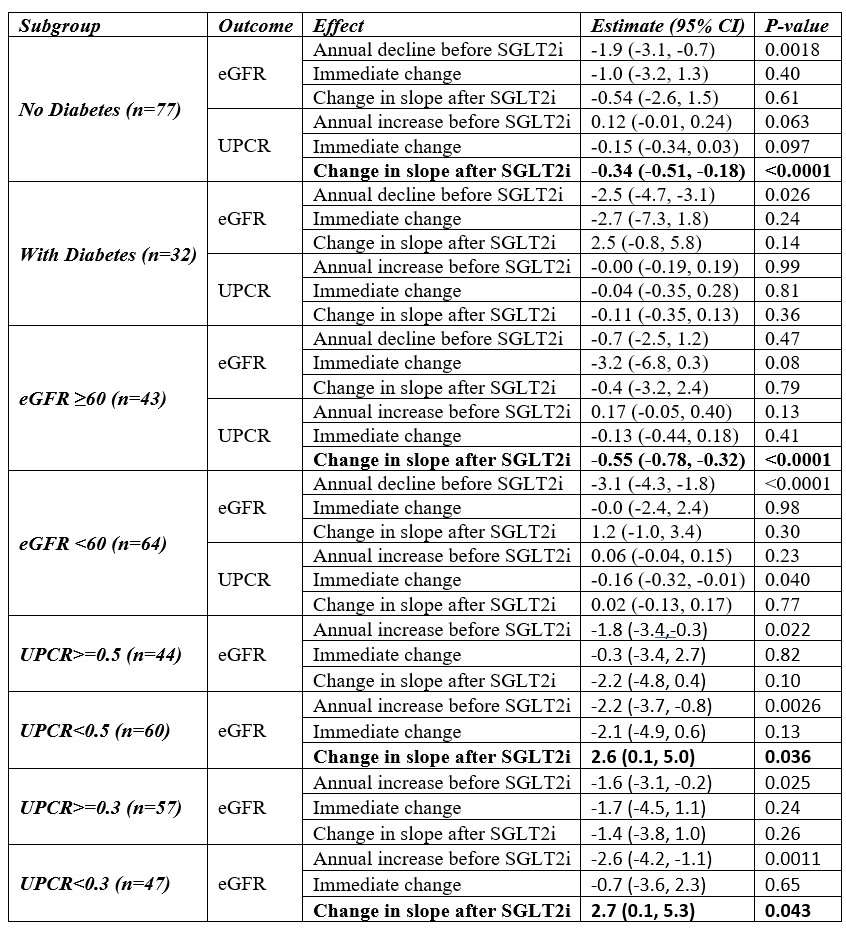 Table 1. Estimated impact of SGLT2 on eGFR and mean Urine Protein-Creatinine ratio.
Table 1. Estimated impact of SGLT2 on eGFR and mean Urine Protein-Creatinine ratio.
.jpg) Table 2. Estimated impact of SGLT2 on eGFR and mean Urine Protein-Creatinine ratio for subgroups based on diabetes mellitus status and baseline UPCR.
Table 2. Estimated impact of SGLT2 on eGFR and mean Urine Protein-Creatinine ratio for subgroups based on diabetes mellitus status and baseline UPCR.
To cite this abstract in AMA style:
Lee J, Fava A, Goldman D, Magder L, Petri M. The Benefits of SGLT2i on GFR Slope and Proteinuria in SLE Depend on Subgroups of Diabetes Mellitus and Baseline eGFR [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-benefits-of-sglt2i-on-gfr-slope-and-proteinuria-in-sle-depend-on-subgroups-of-diabetes-mellitus-and-baseline-egfr/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-benefits-of-sglt2i-on-gfr-slope-and-proteinuria-in-sle-depend-on-subgroups-of-diabetes-mellitus-and-baseline-egfr/
