Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In a prior study, we described an alternative method for subphenotyping RA patients by the sequence of biologic DMARDs (bDMARDs) they receive over time. We identified 3 “clusters” of RA treatment patterns: those prescribed mainly TNF-α inhibitors (TNFi) (“TNFi persisters”), those who start TNFi but switch and predominantly remain on abatacept (“TNFi to abatacept”), and those prescribed multiple bDMARD classes (“multi-bDMARD cyclers”). In this study, we investigated whether: (1) these clusters are observed across healthcare systems, and (2) genetic variants associated with inflammatory arthritis can differentiate these clusters. We hypothesized that RA patients who persisted on TNFi would have a higher genetic risk for RA compared to patients who cycled through multiple bDMARDs.
Methods: The study was performed using data from two large RA cohorts: individuals with RA from the Veteran Affairs Million Veteran Program (MVP) and a Biobank associated with a large multihospital healthcare system. RA patients who initiated TNFi after 2008, had >6 months of follow up, and available genotyping information were included from both sites. We used mixture Markov modeling and Ensemble multi-site clustering to identify patterns of bDMARD use. Using the genotyping data, we calculated previously published multi-disease inflammatory arthritis genetic risk scores (GRS) that includes individual non-HLA genetic risk scores for RA, spondyloarthritis, psoriatic arthritis, lupus, and gout (Knevel, Sci Transl Med, 2020). We compared the distribution of the GRS’ between treatment “clusters” using meta-analysis of Wilcoxon rank-sum tests by Fisher’s method.
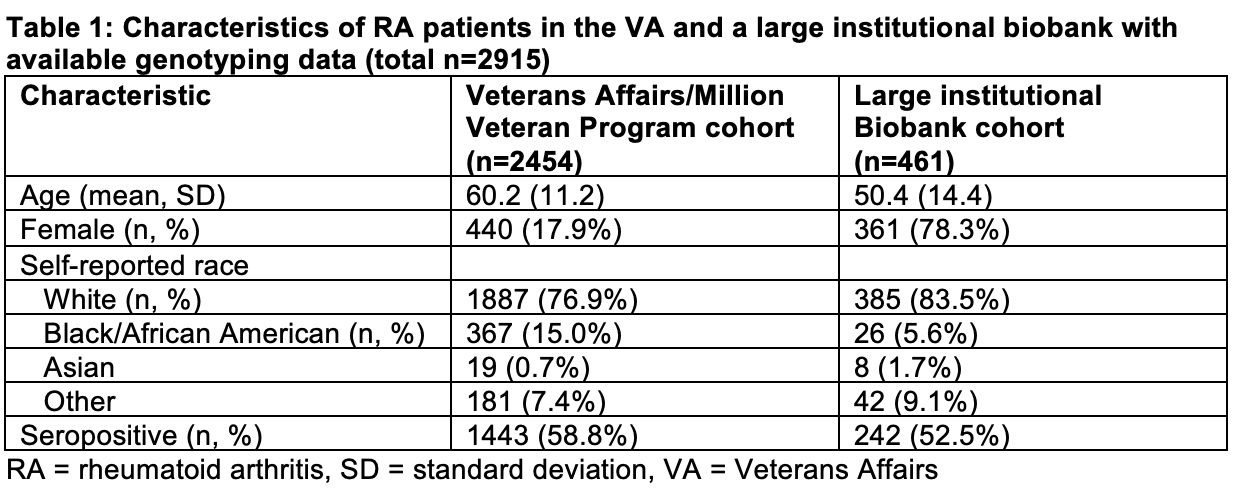
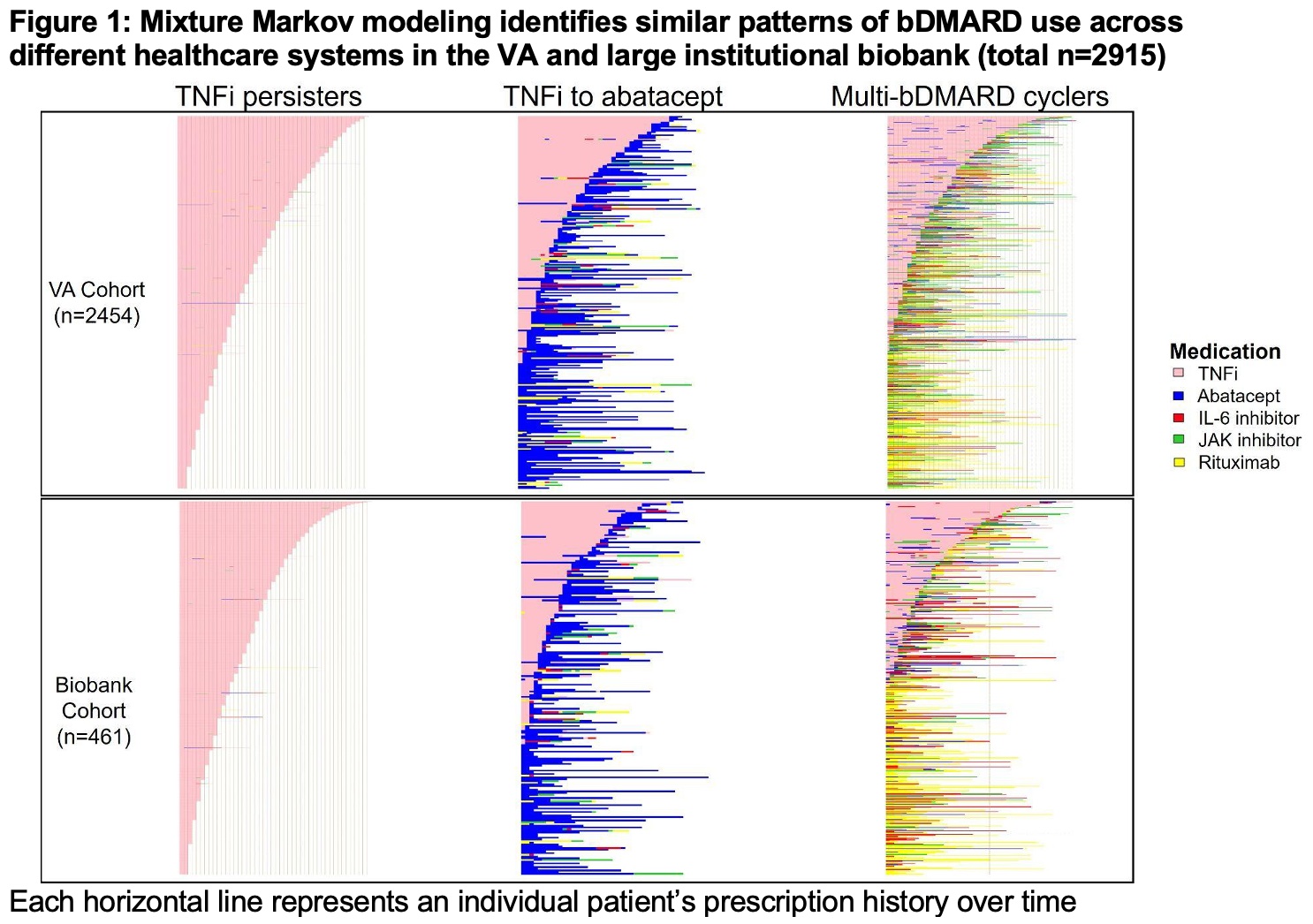
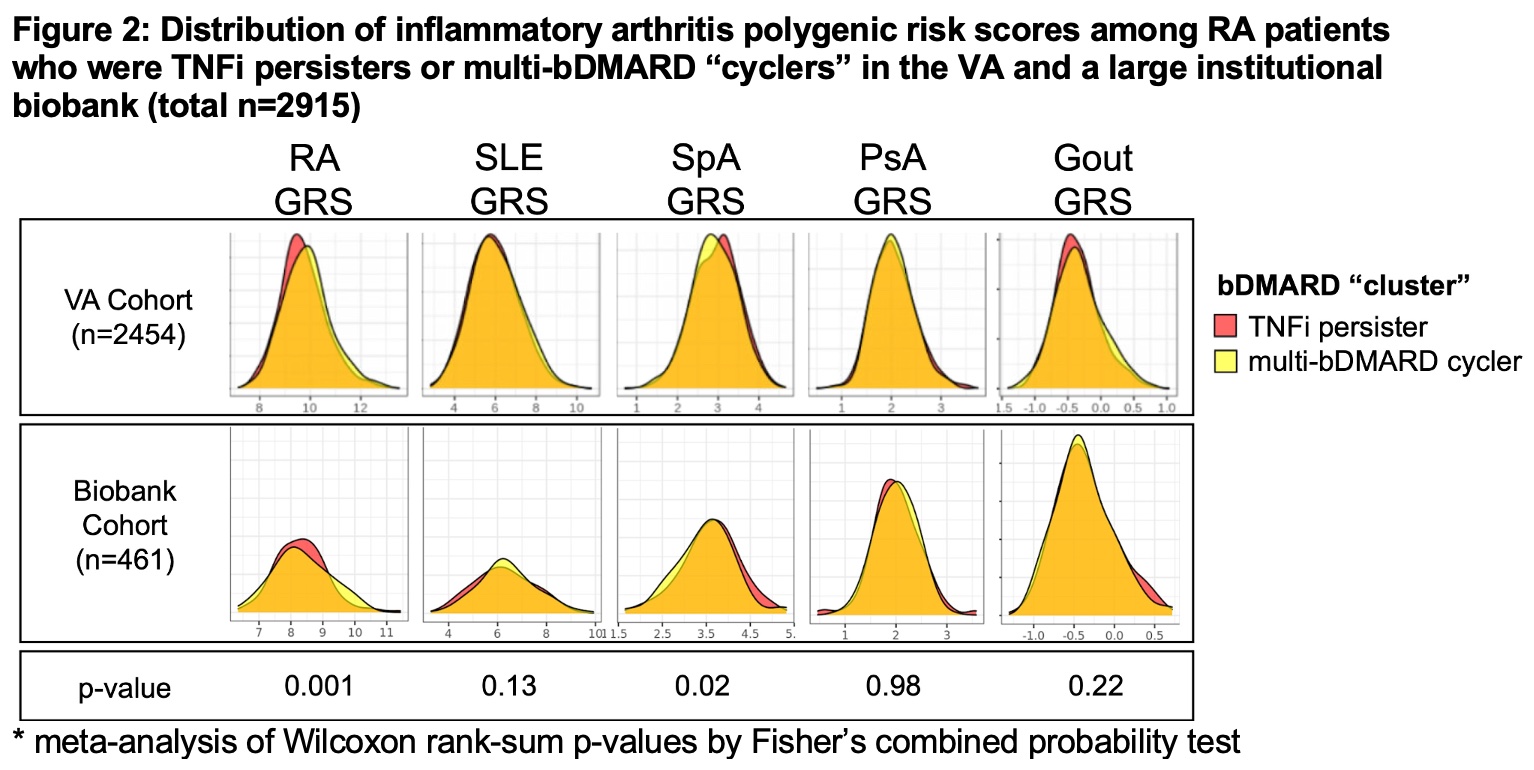
Results: We identified 2,454 patients in the VA cohort (mean age 60, 18% female) and 461 patients in the institutional Biobank (mean age 50, 78% female) (Table 1). We observed similar patterns and “clusters” of bDMARD use in both healthcare systems: TNFi persisters, TNFi to abatacept, and multi-bDMARD cyclers (Figure 1). When comparing genetic risk scores between clusters, we observed that patients who persisted on TNFi had a lower non-HLA RA GRS (p=0.001) and higher SpA GRS (p=0.02) when compared to multi-bDMARD cyclers (Figure 2). There were no other consistent significant genetic differences between the treatment clusters.
Conclusion: In this study comparing RA bDMARD use in different healthcare systems, we observed similar sequencing of bDMARD use, including persistent treatment with TNFi, switching from TNFi and persisting on abatacept, and cycling on multiple bDMARDs. These similarities were observed despite significant differences in underlying RA patient populations, including age and sex. RA patients who persisted on TNFi had non-HLA genetic differences compared to multi-bDMARD cyclers; future studies will incorporate HLA data. These findings suggest that genetics may contribute to understanding response to RA treatment.
To cite this abstract in AMA style:
McDermott G, Xiong X, Knevel R, Cui J, Jeffway M, Panickan V, Cagan A, Sangar R, Posner D, Costa L, Matty R, Karlson E, Ho Y, Cho K, Duan R, Cai T, Liao K. The Associations Between Genetic Factors and Rheumatoid Arthritis Treatment Patterns: Data from Two Large Healthcare Systems [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-associations-between-genetic-factors-and-rheumatoid-arthritis-treatment-patterns-data-from-two-large-healthcare-systems/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-associations-between-genetic-factors-and-rheumatoid-arthritis-treatment-patterns-data-from-two-large-healthcare-systems/