Session Information
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: The RAVE trial established the non-inferiority of rituximab (RTX) vs cyclophosphamide (CYC) for remission induction of ANCA-Associated Vasculitis (AAV). Patients in RAVE were followed for 18 months so little is known regarding the long-term outcomes of these remission induction strategies in real world practice. Here, we evaluated the association of remission induction treatment strategy with the risks of end-stage renal disease (ESRD) and death, two key outcomes in AAV.
Methods: The data source was a consecutive inception cohort of PR3- or MPO-ANCA+ AAV patients assembled in a large healthcare system between 2002 and 2019. Baseline demographics and disease-specific features, including baseline manifestations and laboratory values, were extracted from the electronic health records (EHR) and data warehouse. The primary exposure was RTX- vs CYC-based remission induction regimens, ascertained by manual review. ESRD and vital status, the composite outcome of interest, were ascertained by EHR review as well as linkage to the United States Renal Data System and National Death Index, respectively. A propensity score (PS) model for treatment with RTX vs CYC was created using demographic and disease-specific variables present at baseline, including age, sex, comorbidities, ANCA type, AAV manifestations, and renal function. We first examined the association of RTX vs CYC with the risk of ESRD or death in unadjusted and multivariable adjusted Cox proportional hazard models. We then performed the same analysis after one to many matching RTX users to CYC users by PS.
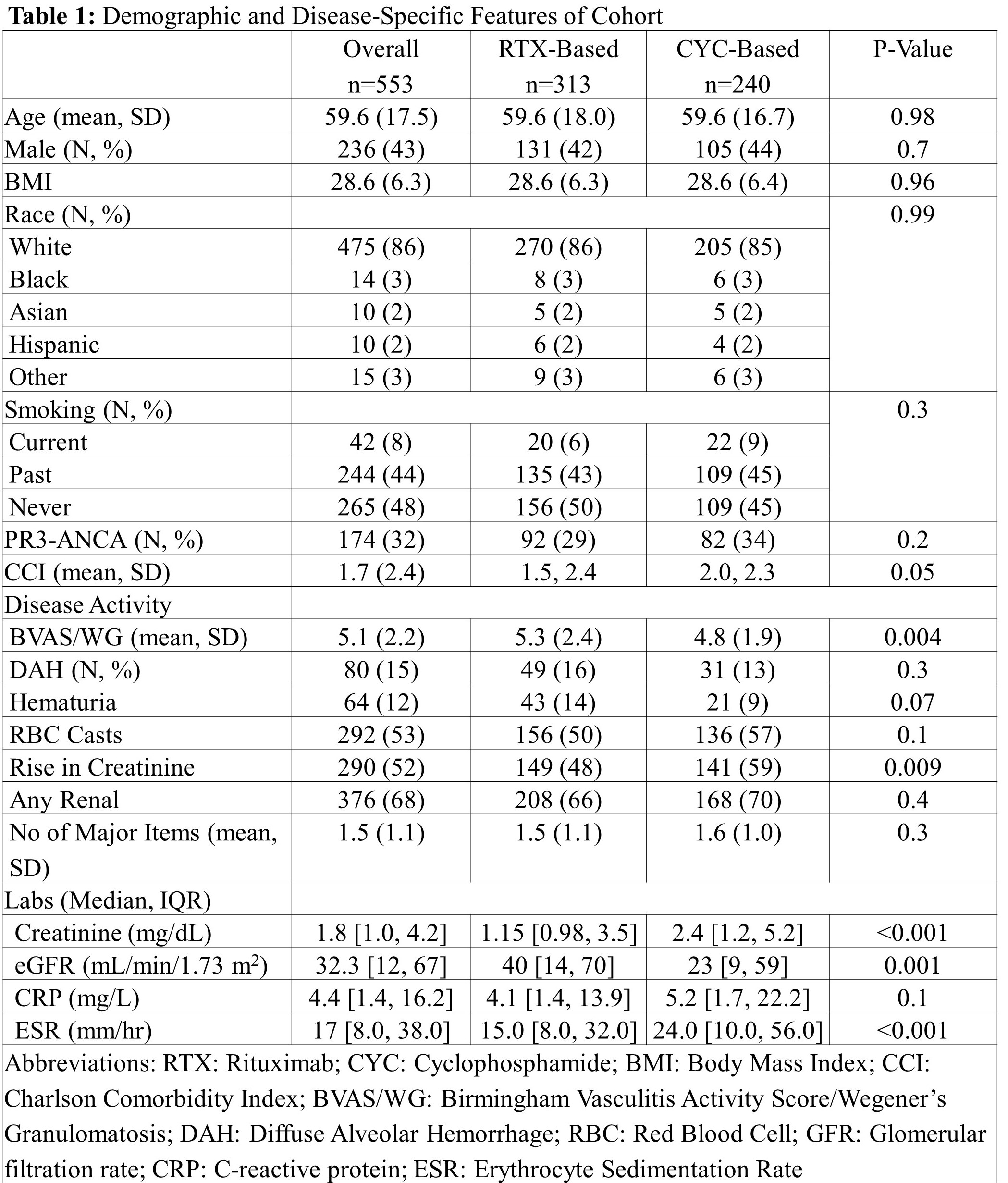
Results: We identified 553 patients who received RTX- or CYC-based regimens (Table 1). The mean (SD) age was 59.6 (17.5) years, 236 (43%) were male, and 475 (86%) were White. The majority were MPO-ANCA+ (379, 68%) and had renal involvement (376, 68%). The mean (SD) BVAS/WG score was 5.1 (2.2). Baseline demographics were similar between RTX- and CYC-users (Table 1). RTX-users had a higher BVAS/WG (5.3 [2.4] vs 4.8 [1.9], p=0.004) and CYC-users had a higher creatinine (2.4 [1.2, 5.2] vs 1.2 [1.0, 3.5], p< 0.001). Over a total follow-up of 64,686 days, there were 182 events (ESRD or death). The incidence of the primary outcome was 3.0/1,000 days in RTX-users vs 2.7/1,000 days in CYC-users (Table 2). In the multivariable adjusted Cox model (n=553), the risk of ESRD or death was similar in RTX- vs CYC-users (1.26, 95% CI 0.89 to 1.79). Our results remained similar in analyses limited to those with renal involvement and those with any major disease. Baseline demographics and disease-specific features were well-balanced after PS-matching. In the PS-matched analysis (n=351), the risk of ESRD or death was similar in RTX- vs CYC-users (1.19, 95% CI 0.75-1.89).
Conclusion: In this large multi-center AAV cohort, there were no significant differences in the risk of ESRD or death associated with RTX- vs CYC-based therapy for remission induction. Our observations remained consistent in analyses using propensity score matching to robustly address confounding by indication. These results will inform the care of patients with AAV for whom there remains some uncertainty regarding initial choice of immunosuppression.
To cite this abstract in AMA style:
Wallace Z, Fu X, Cook C, Zhang Y, Stone J, Choi H. The Association of Rituximab- vs. Cyclophosphamide-Based Remission Induction Strategies with Risk of End-Stage Renal Disease and Death in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-association-of-rituximab-vs-cyclophosphamide-based-remission-induction-strategies-with-risk-of-end-stage-renal-disease-and-death-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-of-rituximab-vs-cyclophosphamide-based-remission-induction-strategies-with-risk-of-end-stage-renal-disease-and-death-in-anca-associated-vasculitis/