Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
To determine the association of body mass index (BMI) with incident COVID-19 infection in patients with inflammatory arthritis (IA) using biologic disease-modifying anti-rheumatic drugs (bDMARDs) and describe the demographic and clinical characteristics of patients with severe COVID-19.
Methods:
This was a prospective inception cohort study of IA patients ≥ 21 years old initiating a bDMARD after July 2016 across 6 public-sector hospitals in Singapore. Data were collected via face-to-face questionnaires and abstracted from electronic medical records. Baseline characteristics were compared using chi-square test for categorical variables; and t-test and Mann-Whitney U test for continuous variables for normal and non-normal distributions respectively. The association of BMI with COVID-19 infection was analyzed using multivariate logistic regression, adjusting for demographics and other baseline characteristics.
Results:
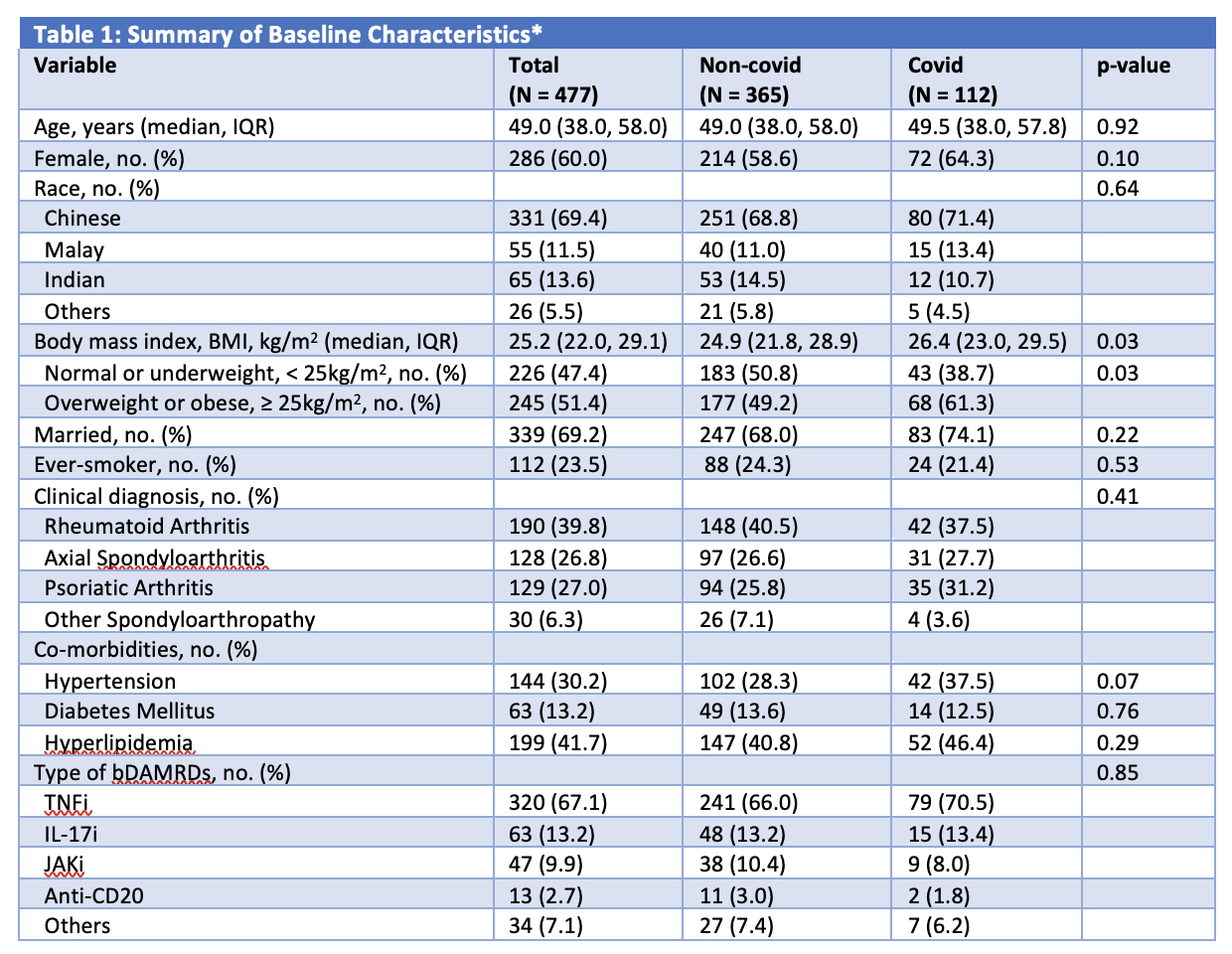
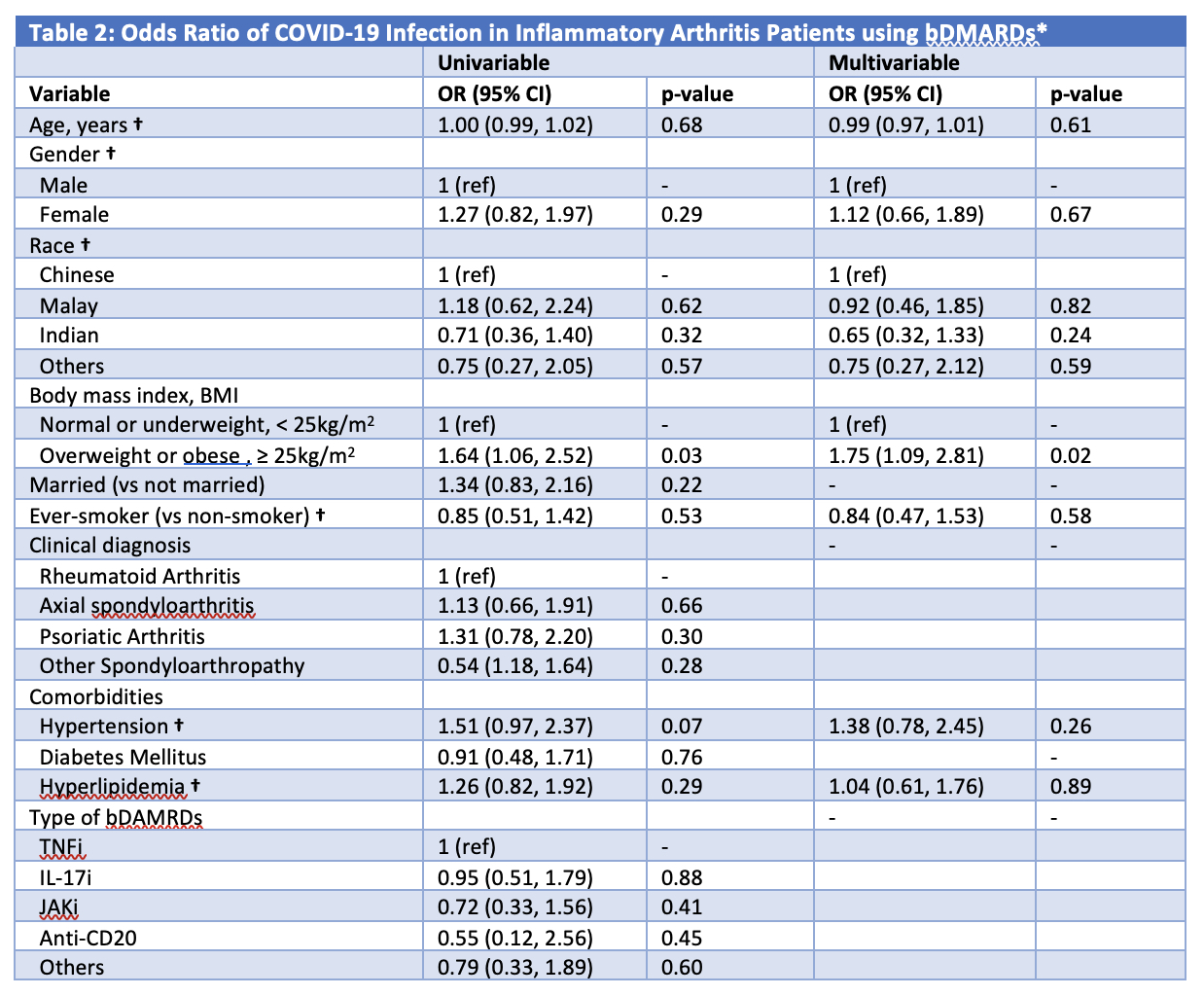
477 patients (60.0% female, 69.4% Chinese) with median (IQR) age of 49.0 (38.0, 58.0)years were included. 39.8% had rheumatoid arthritis, 27.0% had psoriatic arthritis and 33.1% had a spondyloarthropathy. The majority of patients (67.1%) were on tumor necrosis factor inhibitors (TNFi). 148 (31.0%) patients had incident SARS-CoV-2 infection. Compared to patients not infected with COVID-19, those who contracted COVID-19 had a higher BMI (24.9 vs 26.4 kg/m2, p = 0.03) (Table 1). Having a BMI of ≥ 25 kg/m2 (odds ratio [OR] 1.75; 95% CI (1.09, 2.81), p = 0.02) was independently associated with COVID-19 infection, after adjusting for age, sex, race, smoking status and co-morbidities (Table 2).
Severe COVID-19 was defined as infections resulting in hospitalization and/or death. Three (2.0%) of 148 patients had severe COVID-19 infection requiring hospitalization. All were male and two were Chinese. Two had rheumatoid arthritis while one had axial spondyloarthritis. All three had co-morbidities of hypertension and hyperlipidemia, and two had diabetes mellitus. At the time of severe COVID-19 infection, the patients were respectively on golimumab, tofacitinib and adalimumab biosimilar (Amgevita®).
Conclusion:
Higher BMI was associated with incident COVID-19 infection in this cohort. This could be due to upregulation of angiotensin-converting enzyme 2 (ACE2) receptors found in adipose tissues and impaired viral elimination as a result of disease-related and/ or obesity-induced blunted immune response and chronic inflammation [1]. Severe SARS-CoV-2 infections in this cohort were rare.Optimization of BMI may contribute to reducing risk and severity of COVID-19 infection.
[1] Demeulemeester, F., de Punder, K., van Heijningen, M., & van Doesburg, F. (2021). Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review.Cells,10(4), 933. https://doi.org/10.3390/cells10040933
bDMARD: biological disease modifying anti-rheumatic drug
TNFi, Tumor necrosis factor inhibitors – adalimumab (Humira®), golimumab, infliximab, etanercept, adalimumab biosimilar (Amgevita®) and infliximab biosimilar (Remsima®); IL_17i, Interleukin_17 inhibitor – secukinumab, ixekizumab; JAKi, Janus kinase inhibitor – tofacitinib, baricitinib; Anti-CD20 – rituximab, rituximab biosimilar (Truxima®); Others include tocilizumab (Interleukin (IL)-6 inhibitor), gulselkumab and ustekinumab (IL_12/23 inhibitors) and abatacept (Cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig)).
* Variables were included in the multivariable model if they were significant at P ≤ 0.2 on univariate analysis. † Included in the multivariable model despite not statistically significant in the univariable analysis due to significant correlation with BMI and need to be adjusted for.
TNFi, Tumor necrosis factor inhibitors – adalimumab (Humira®), golimumab, infliximab, etanercept, adalimumab biosimilar (Amgevita®) and infliximab biosimilar (Remsima®); IL_17i, Interleukin_17 inhibitor – secukinumab, ixekizumab; JAKi, Janus kinase inhibitor – tofacitinib, baricitinib; Anti-CD20 – rituximab, rituximab biosimilar (Truxima®); Others include tocilizumab (IL-6 inhibitor), gulselkumab and ustekinumab (IL_12/23 inhibitors) and abatacept (Cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig)).
To cite this abstract in AMA style:
Dhanasekaran P, Ma M, Lahiri M, Koh L, Angkodjojo S, Khor A, Liu J, Wong S, Yeo S. The Association of Body Mass Index with SARS-CoV-2 Infection (COVID-19) in Patients with Inflammatory Arthritis on Biologic Disease Modifying Anti-Rheumatic Drugs: Results from the Singapore National Biologics Register [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-association-of-body-mass-index-with-sars-cov-2-infection-covid-19-in-patients-with-inflammatory-arthritis-on-biologic-disease-modifying-anti-rheumatic-drugs-results-from-the-singapore-national/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-of-body-mass-index-with-sars-cov-2-infection-covid-19-in-patients-with-inflammatory-arthritis-on-biologic-disease-modifying-anti-rheumatic-drugs-results-from-the-singapore-national/