Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: High dose tumour necrosis factor inhibitor (TNFi) drugs are associated with an increased serious infection (SI) risk[1]. It is feasible that high biologic levels predict dose-dependent adverse events such as SI. No registries have systematically evaluated the effect of drug levels on infection risk. The objective was to assess the effect of biologic drug levels in rheumatoid arthritis (RA) patients on (i) all infections (AI) (ii) SI (infections requiring hospitalization, IV antibiotics or lead to death) Methods:
Patients recruited to both the British Society for Rheumatology Biologics Register-RA (safety data) and the Biologics in RA Genetics & Genomics Syndicate (serological samples) were included. Both are large national prospective RA cohorts. Biologic drug levels were measured at 3/6/12 months after biologic initiation and stratified as low/normal or high drug levels (HL) as per thresholds defined using concentration-effect curves for each drug. The risk of first and total infections within the first year was analysed. Events occurring on drug or within 90 days of last dose were included. The risk of an event was compared between low/normal vs. HL groups using Cox proportional-hazard models. Factors affecting both drug levels and infection risk were adjusted for in the models. Results:
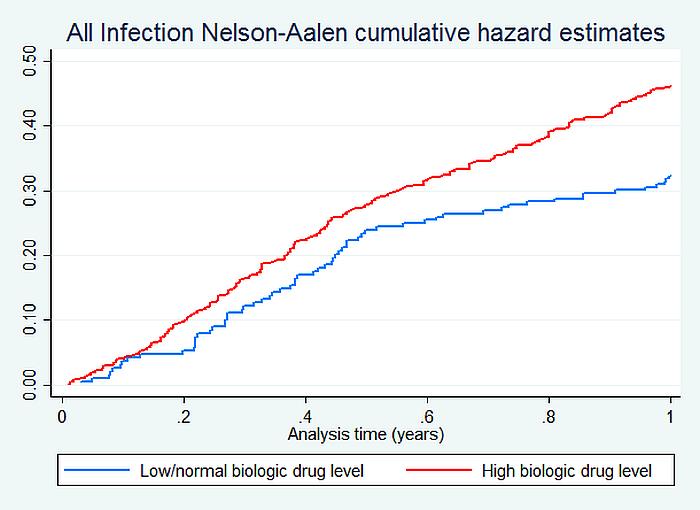
703 patients (286 etanercept, 179 adalimumab, 120 certolizumab, 104 tocilizumab and 14 infliximab) had clinical data and serological samples. 74% were women, mean (SD) age 58 (12) years, on a first biologic (89%). The crude rate/1000 pyrs was 314 and 464 for AI; 54 and 76 for SI in the low/normal and HL groups respectively. The adjusted hazard ratio for AI within the first year differed significantly between the two groups with the HL group having 50 percent higher risk of AI (HR: 1.51; 95% CI: 1.14, 2.01) (table). The most common types of AI in the HL group were lower (34%) and upper (16%) respiratory tract infections, urinary tract infections (15%), skin infections including shingles (8%).
|
|
Low/ normal drug level (n=241) |
High drug levels (n=462) |
|
All events with follow up censored at 1 year (95% CI) |
||
|
All infections (n) |
63 |
232 |
|
Crude rate (/1000 pyrs) |
314 (245, 401) |
464 (408, 528) |
|
Unadjusted HR |
Ref |
1.44 (1.09, 1.91)* |
|
Adjusted HR † |
Ref |
1.51 (1.14, 2.01)* |
|
Serious infections (n) |
11 |
38 |
|
Crude rate (/1000 pyrs) |
54 (30, 98) |
76 (55, 104) |
|
Unadjusted HR |
Ref |
1.36 (0.70, 2.67) |
|
Adjusted HR† |
Ref |
1.17 (0.58, 2.30) |
|
First event within 1st year (95% CI) |
||
|
All infections (n) |
46 |
150 |
|
Crude rate (1000 pyrs) |
229 (172, 256) |
300 (256, 352) |
|
Unadjusted HR |
Ref |
1.24 (0.89, 1.73) |
|
Adjusted HR † |
Ref |
1.26 (0.91, 1.78) |
|
Serious infections (n) |
6 |
22 |
|
Crude rate (/1000 pyrs) |
29 (13, 67) |
44 (28, 67) |
|
Unadjusted HR |
Ref |
1.39 (0.56, 3.44) |
|
Adjusted HR† |
Ref |
1.26 (0.50, 3.16) |
*p<0.05 †Adjusted for age, gender, DAS score, methotrexate use
Image/graph:
Conclusion:
RA patients with high biologic drug levels have a higher risk of infection. Monitoring drug levels may be helpful in prediction of infection. In disease remission patients with high drug levels, biologic dose tapering may lower infection risk.
References: (1)Singh JA, Cameron C et al. Lancet 2015;368;258-265
Acknowledgements: Funded by MRC CiC award & BMA Doris Hillier grant to MJ, AB, WGD, KLH.
To cite this abstract in AMA style:
Jani M, Dixon WG, Lunt M, De Cock D, Isaacs J, Morgan A, Watson K, Wilson AG, Barton A, Hyrich KL. The Association of Biologic Drug-Levels with Infection Risk: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-association-of-biologic-drug-levels-with-infection-risk-results-from-the-british-society-for-rheumatology-biologics-register-for-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-of-biologic-drug-levels-with-infection-risk-results-from-the-british-society-for-rheumatology-biologics-register-for-rheumatoid-arthritis/