Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Basic Science Poster
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Heterogeneity is a hallmark of psoriatic arthritis (PsA), which is reflected in diverse clinical, imaging and molecular features, various disease courses and treatment responses. We hypothesized that specific molecular markers underlie the various imaging manifestations of PsA. The study objective was to identify imaging sub-phenotypes in patients with PsA and determine their association with whole blood mRNA expression markers.
Methods: 55 patients with PsA ready to initiate treatment for active disease were prospectively recruited. An ultrasound assessment of the extent of musculoskeletal inflammation in 64 joints, 34 tendons and 16 entheses was performed. Sonographic inflammation (in greyscale and Doppler) of the following domains was graded for: a) synovitis; b) peritenonitis; c) tenosynovitis; and d) enthesitis. A global inflammatory score was calculated for each tissue domain. Peripheral blood was profiled with RNAseq, and gene expression data were obtained. Analyses were performed in two stages: 1) Unsupervised cluster analysis was performed to define imaging sub-phenotypes in PsA that reflected the predominant tissue involved; 2) Principal component analysis was used to determine the association between imaging-defined clusters and peripheral blood gene expression profile. Pathway enrichment analysis was performed to identify underlying mechanisms that characterize individual imaging clusters.
Results: The patients could be divided into 3 groups based on unsupervised hierarchical clustering of images indicating the predominant involved tissue (Figure 1): 1) Synovitis predominant (N=31 [56%]); 2) Enthesitis predominant (N=13 [24%]); 3) Peritenonitis predominant (N=11 [20%]). There were no significant differences in the demographics, duration of PsA and psoriasis characteristics between the clusters (Figure 1). The primary differences between the clusters were related to the severity of clinical joint ad entheseal involvement. Unsupervised clustering of gene expression data identified three clusters that partially overlapped with the imaging clusters (Figure 2). Overall, 344 genes were differentially expressed (p< 0.05) in two of the three comparisons between the imaging clusters. Enriched differential pathways included: toll-like receptor cascade, cytokine (TNF, IL-23, TGF-b) signaling, VEGF signaling, complement cascade and integrin signaling (Figure 3).
Conclusion: We identified three different imaging clusters based on the predominant tissue involved in patients with active PsA. Distinct biologic pathways may underlie these imaging clusters seen in PsA.
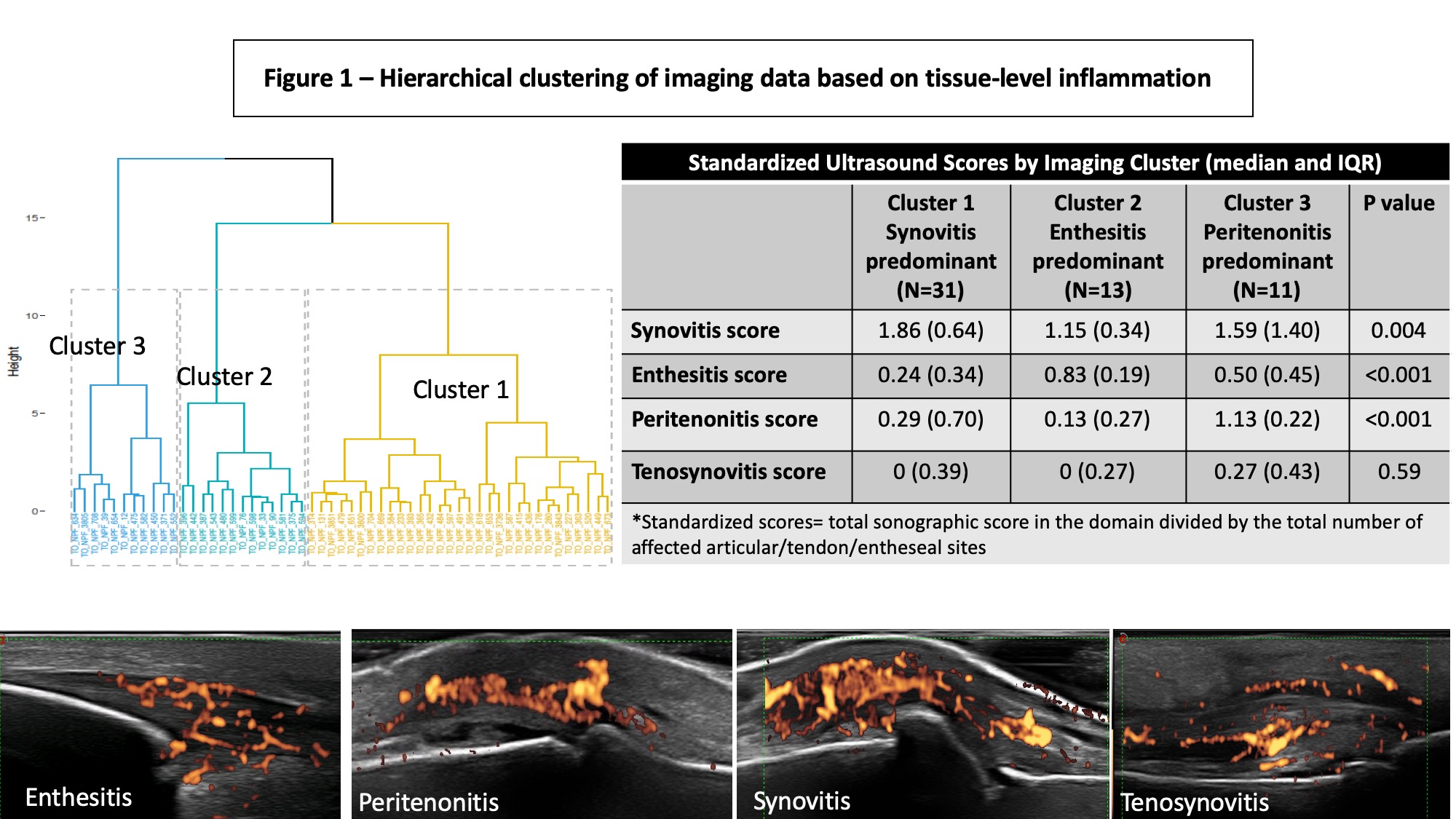 Figure 1 -Hierarchical clustering of imaging data based on tissue-level inflammation
Figure 1 -Hierarchical clustering of imaging data based on tissue-level inflammation
 Figure 2 – Gene Expression Data by Imaging Clusters
Figure 2 – Gene Expression Data by Imaging Clusters
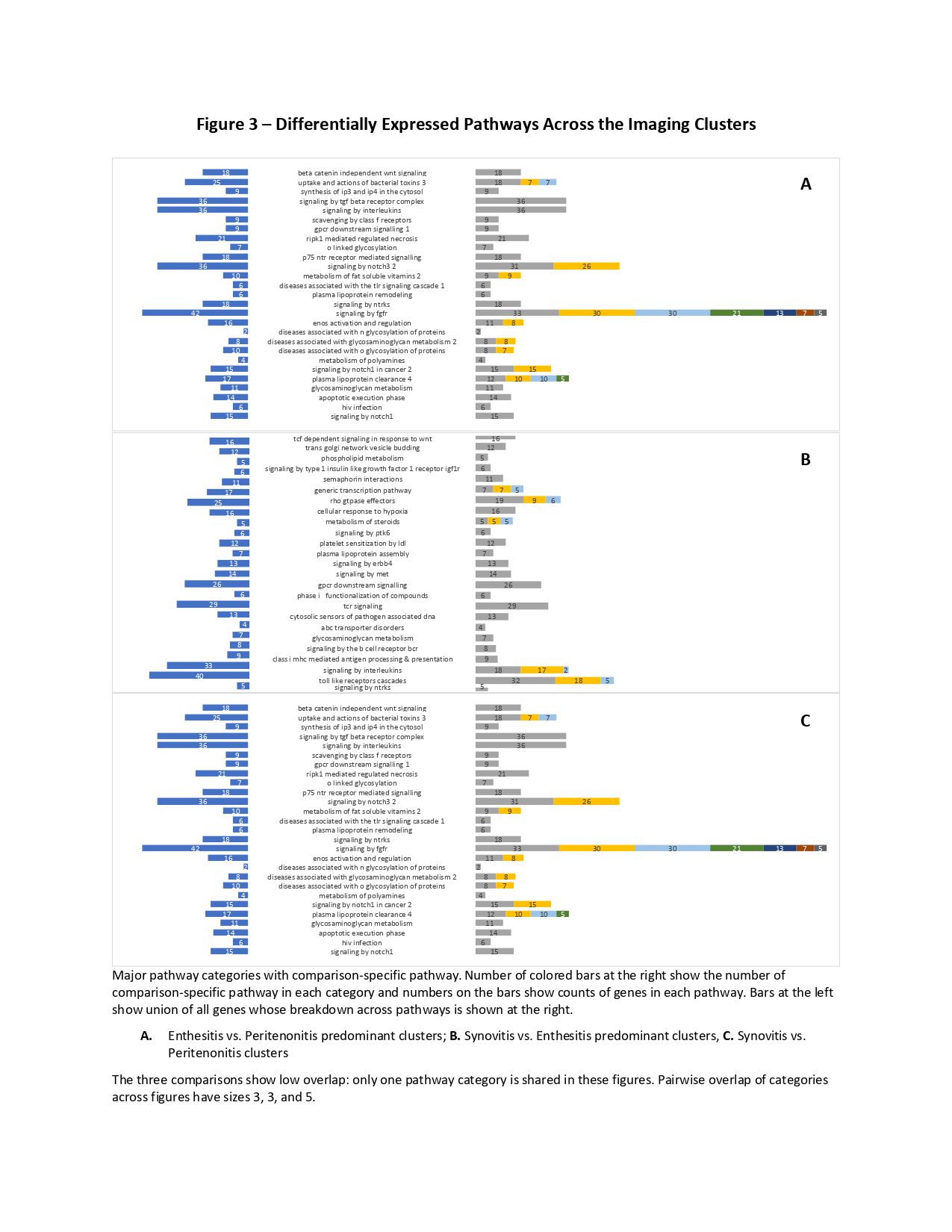 Figure 3 – Differentially Expressed Pathways Across the Imaging Clusters
Figure 3 – Differentially Expressed Pathways Across the Imaging Clusters
To cite this abstract in AMA style:
Eder L, Quan L, Rahmati S, Eshed I, Rahman P, Jurisica I, Chandran V. The Association Between Imaging Sub-phenotypes of Psoriatic Arthritis and Gene Expression Profiles [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-association-between-imaging-sub-phenotypes-of-psoriatic-arthritis-and-gene-expression-profiles/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-between-imaging-sub-phenotypes-of-psoriatic-arthritis-and-gene-expression-profiles/
