Session Information
Session Type: Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: Advances in prevention, testing, and treatment of COVID-19 have contributed to less severe outcomes in the general population, but whether outcomes have improved for patients with systemic autoimmune rheumatic disease (SARD) remains unclear. Previous studies of temporal trends in COVID-19 outcomes among SARD patients were performed prior to the wide availability of vaccines and the Omicron wave. We aimed to investigate temporal trends in incidence and severity of COVID-19 among SARD patients.
Methods: We performed a retrospective cohort study investigating temporal trends in COVID-19 outcomes among SARD patients with COVID-19 from March 1, 2020 to January 31, 2022 at a large healthcare system. COVID-19 cases were systematically identified using electronic query of PCR results supplemented by physician referral of patients with testing outside of the healthcare system. Presence of SARD, demographic data, comorbidities, vaccination status, and medications were confirmed by chart review. We tabulated COVID-19 case counts and the subset with severe outcomes, defined as hospitalization, mechanical ventilation, or death within 30 days following the positive test. We divided calendar time into periods based on changes in viral epidemiology and care advances: T1: March 1 – June 30, 2020 (Early COVID-19); T2: July 1, 2020 – January 31, 2021 (Early treatment); T3: February 1 – June 30, 2021 (Early vaccination); T4: July 1 – December 16, 2021 (Delta wave); T5: December 17, 2021 – January 31, 2022 (Omicron wave). We compared the proportion of patients with severe COVID-19 by calendar period as well as vaccination status. We used logistic regression to estimate the odds ratios for severe COVID-19 for each period compared to T1 as the reference.
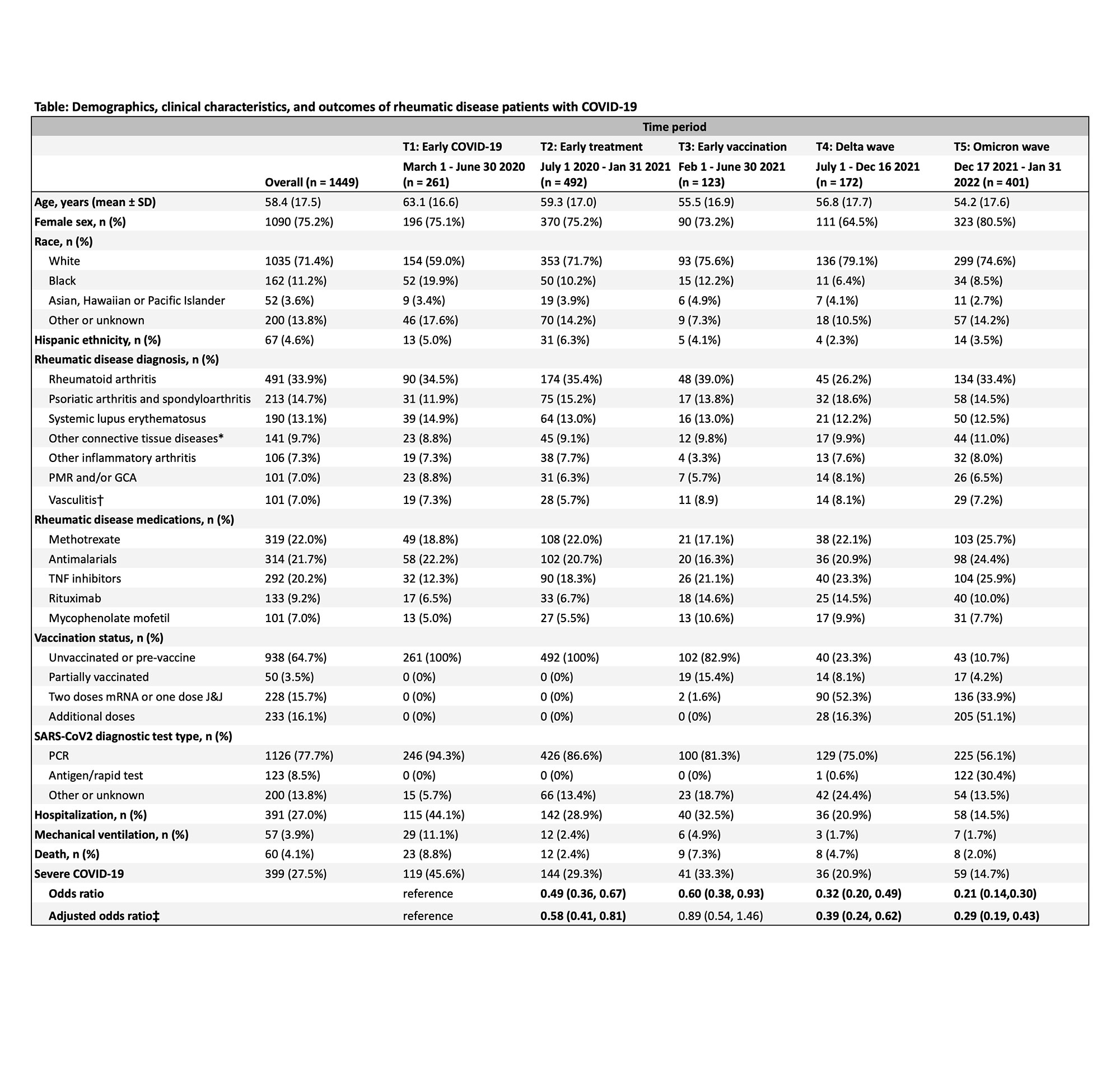
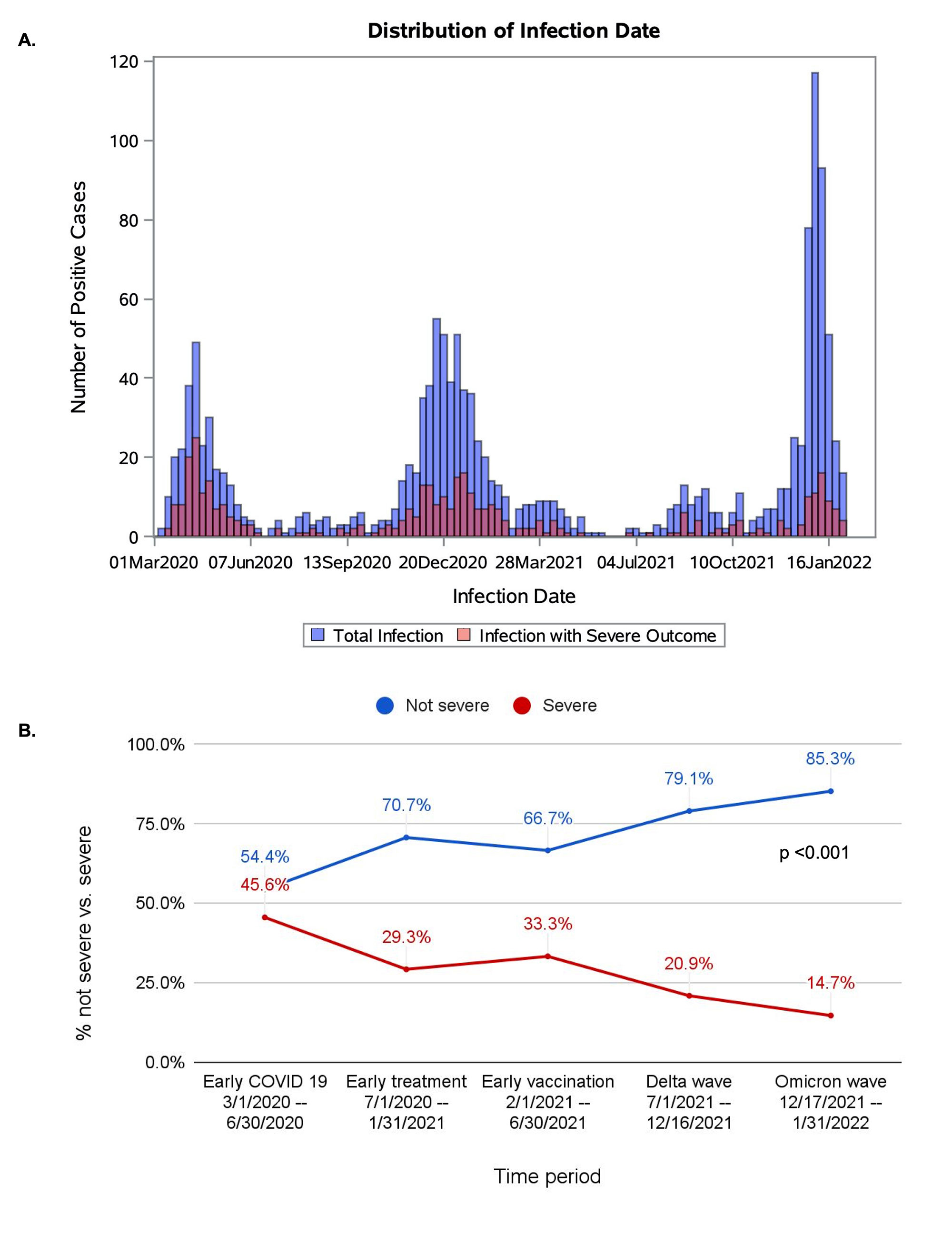
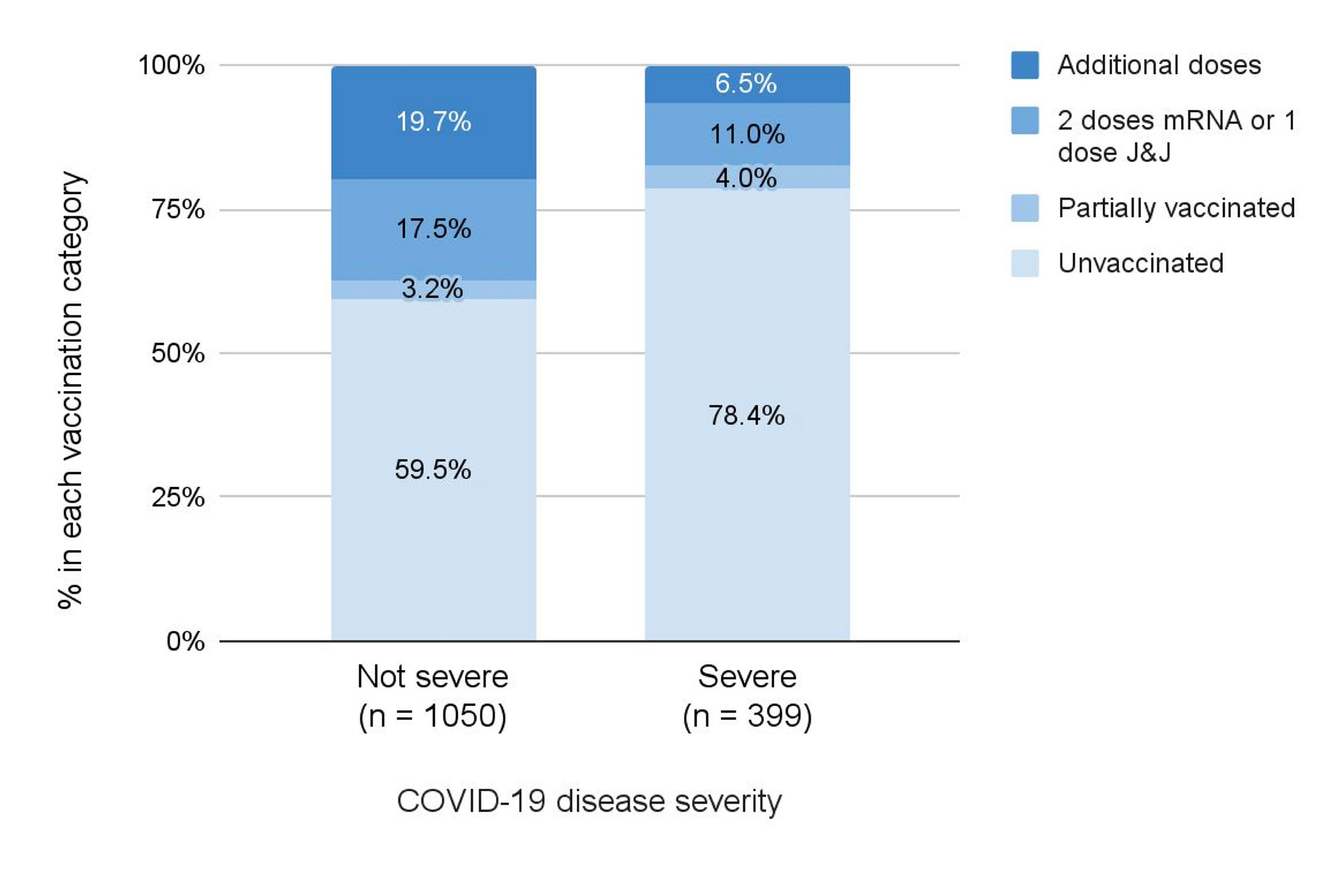
Results: We identified 1449 SARD patients with COVID-19 (mean age 58.4 years, 75.2% female, 33.9% with RA). There were 399 (27.5%) cases of severe COVID-19 (391 [27.0%] hospitalized and 60 [4.1%] deaths) (Table). Figure 1 shows the number of total cases and severe cases over calendar time and proportion of severe cases in each period. The proportion of severe COVID-19 outcomes was lower over calendar time: 45.6% in T1, 29.3% in T2, 33.3% in T3, 20.9% in T4, and 14.7% in T5 (p for trend < 0.001). The adjusted odds ratio of severe COVID-19 in T5 (Omicron wave) was 0.29 (95% CI 0.19-0.43) compared to T1. However, the absolute number of severe COVID-19 cases during the peak of the Omicron variant wave was similar to the peaks of other waves. Unvaccinated patients made up a higher proportion of severe cases compared to not severe cases (78.4% vs. 59.5%) (Figure 2).
Conclusion: The proportion of SARD patients with COVID-19 experiencing severe outcomes has greatly diminished since early in the pandemic, particularly in the most recent time periods. These results suggest that advances in prevention, diagnosis, and treatment of COVID-19 may have improved outcomes among patients with SARD. However, other factors such as hospital capacity, depletion of susceptible people, shielding behaviors, and virulence of viral variants are also likely to contribute to the reduced odds of severe COVID-19 over time.
*Includes systemic sclerosis, inflammatory myositis, Sjogren’s syndrome, undifferentiated connective tissue disease, mixed connective tissue disease, antiphospholipid syndrome (without SLE).
†Includes ANCA vasculitis, Takayasu’s arteritis, Kawasaki disease, Behçet’s disease, polyarteritis nodosa, other vasculitis.
‡ Adjusted for age, sex, and race.
A. Case counts of SARS-CoV_2 infections over calendar time with total infections shown in blue and infections with severe outcomes in red. B. Proportion of cases with severe and non-severe outcomes in each time period. P value for trend.
mRNA, messenger ribonucleic acid; J&J, Johnson & Johnson / Janssen.
To cite this abstract in AMA style:
Kawano Y, Patel N, Wang X, Cook C, Vanni K, Kowalski E, Banasiak E, Qian G, Diiorio M, Hsu T, Weinblatt M, Todd D, Wallace Z, Sparks J. Temporal Trends in COVID-19 Outcomes Among Patients with Systemic Autoimmune Rheumatic Diseases: From the First Wave to Omicron [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/temporal-trends-in-covid-19-outcomes-among-patients-with-systemic-autoimmune-rheumatic-diseases-from-the-first-wave-to-omicron/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/temporal-trends-in-covid-19-outcomes-among-patients-with-systemic-autoimmune-rheumatic-diseases-from-the-first-wave-to-omicron/