Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Telitacicept is a novel recombinant fusion protein constructed with the extracellular domain of the human transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptor and the Fc domain of human immunoglobulin (Ig) G1 targeting and neutralizing B lymphocyte stimulator (BLyS) and a proliferating-inducing ligand (APRIL). This phase 3 study evaluated the efficacy and safety of Telitacicept 160 mg versus placebo in combination with standard therapy in SLE patients.
Methods: This was a 52-week, randomized, double-blind, placebo-controlled, phase 3 trial (NCT04082416). SLE patients aged 18 to 65 years with positive ANA and/or anti-dsDNA and a SELENA-SLEDAI score ≥8 were randomized 1:1 to receive either Telitacicept 160 mg or placebo subcutaneously once weekly in combination with standard therapy for 52 weeks. The primary endpoint was the SLE Responder Index 4 (SRI4) response rate at Week 52. SRI4 response was defined as: ≥4 points reduction from baseline in SELENA-SLEDAI score, and no new BILAG A organ domain score or ≤ 2 new BILAG B domain scores compared with baseline, and no worsening (an increase of < 0.30 points from baseline) in Physician’s Global Assessment (PGA).
Results: A total of 335 patients were randomized to receive Telitacicept 160 mg (n=167) or placebo (n=168). Baseline demographics and disease characteristics were similar between treatment groups. Of randomized patients, 236 (70.4%) completed 52 weeks of treatment. The primary endpoint at week 52 was met, with significantly greater proportion of patients in Telitacicept 160 mg group achieving SRI4 response as compared with the placebo group (82.6% vs. 38.1%, p< 0.001). The SRI4 response was significantly greater in the Telitacicept group as compared with the placebo group as early as Week 4, and the significant difference was sustained up to week 52 (p < 0.01 for all time points) (Figure 1).
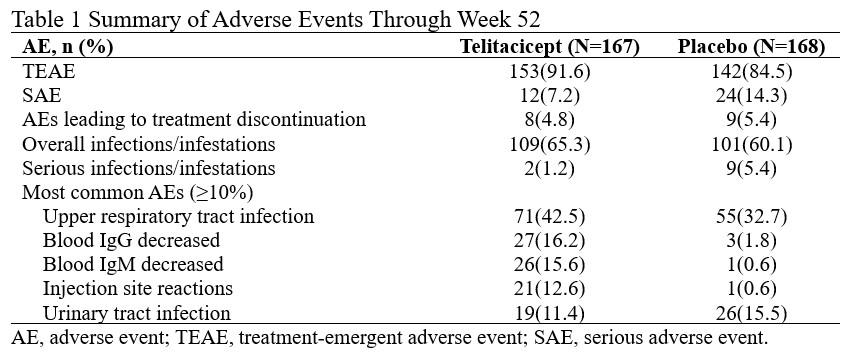
91.6% of subjects in the Telitacicept group and 84.5% in the placebo group had treatment-emergent adverse events (TEAEs). There were 17 serious adverse events (SAEs) in 12 subjects (7.2%) treated with Telitacicept and 36 SAEs in 24 subjects (14.3%) treated with placebo. No death occurred during the study. Rates of overall infections/infestations were similar between Telitacicept and placebo groups. The most common AEs (≥10%) with Telitacicept were upper respiratory tract infection, blood IgG decreased, blood IgM decreased, injection site reactions, and urinary tract infection (Table 1).
Conclusion: This phase 3 trial met the primary endpoint. Telitacicept was well tolerated in SLE patients.
To cite this abstract in AMA style:
Wu D, Li J, Xu D, Wang L, Fang J, Ross D, Zhang F. Telitacicept, a Human Recombinant Fusion Protein Targeting B Lymphocyte Stimulator (BlyS) and a Proliferation-Inducing Ligand (APRIL), in Systemic Lupus Erythematosus (SLE): Results of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/telitacicept-a-human-recombinant-fusion-protein-targeting-b-lymphocyte-stimulator-blys-and-a-proliferation-inducing-ligand-april-in-systemic-lupus-erythematosus-sle-results-of-a-phase-3-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/telitacicept-a-human-recombinant-fusion-protein-targeting-b-lymphocyte-stimulator-blys-and-a-proliferation-inducing-ligand-april-in-systemic-lupus-erythematosus-sle-results-of-a-phase-3-study/