Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The centrality of Toll-like receptor 7 (TLR7) to the pathogenesis of systemic lupus erythematosus (SLE) was recently underscored by a report that high TLR7 gene expression drives autoantibody production in SLE (Wang et al., 2019)and reaffirmed by a report that TLR7 gain-of-function mutations confer susceptibility to SLE (Brown et al., 2022). It was also reported that an antagonistic anti-mouse TLR7 monoclonal antibody (mAb) improves survival and inhibits autoantibody production in NZBWF1 mice, which spontaneously develop lupus (Murakami et al., 2021). We have identified DS-7011a, an antagonistic anti-human TLR7 mAb, and examined its preclinical pharmacological activity and safety. We have also examined the expression of the TLR7 gene signature (GS) as an indicator of target engagement in NZBWF1 mice.
Methods: The species cross-activity and the binding specificity of DS-7011a were evaluated using Lenti-X 293 T cells overexpressing either TLR7 ortholog proteins of several animal species or human TLR family proteins. To evaluate the modulation of cytokine and antibody production by anti-TLR7 antagonistic antibodies, human or mouse peripheral blood mononuclear cells (PBMCs) were stimulated with CL264 or ssRNA9.2s, which are TLR7 agonists. A surrogate antagonistic anti-mouse TLR7 mAb was administered to NZBWF1 mice intraperitoneally at 0.2 mg/mouse once a week from Week 21 to Week 30 of age to study the impact on the lupus model along with the modulation of TLR7 GS. TLR7 GS was defined using public microarray data of mouse macrophages treated with R848, a TLR7 agonist. TLR7 GS scores were calculated using blood gene expression data from NZBWF1 mice treated with anti-TLR7 mAb or isotype control antibody or left untreated. To evaluate safety, DS-7011a was administered intravenously once every 2 weeks for 3 months to cynomolgus monkeys at doses up to 400 mg/kg.
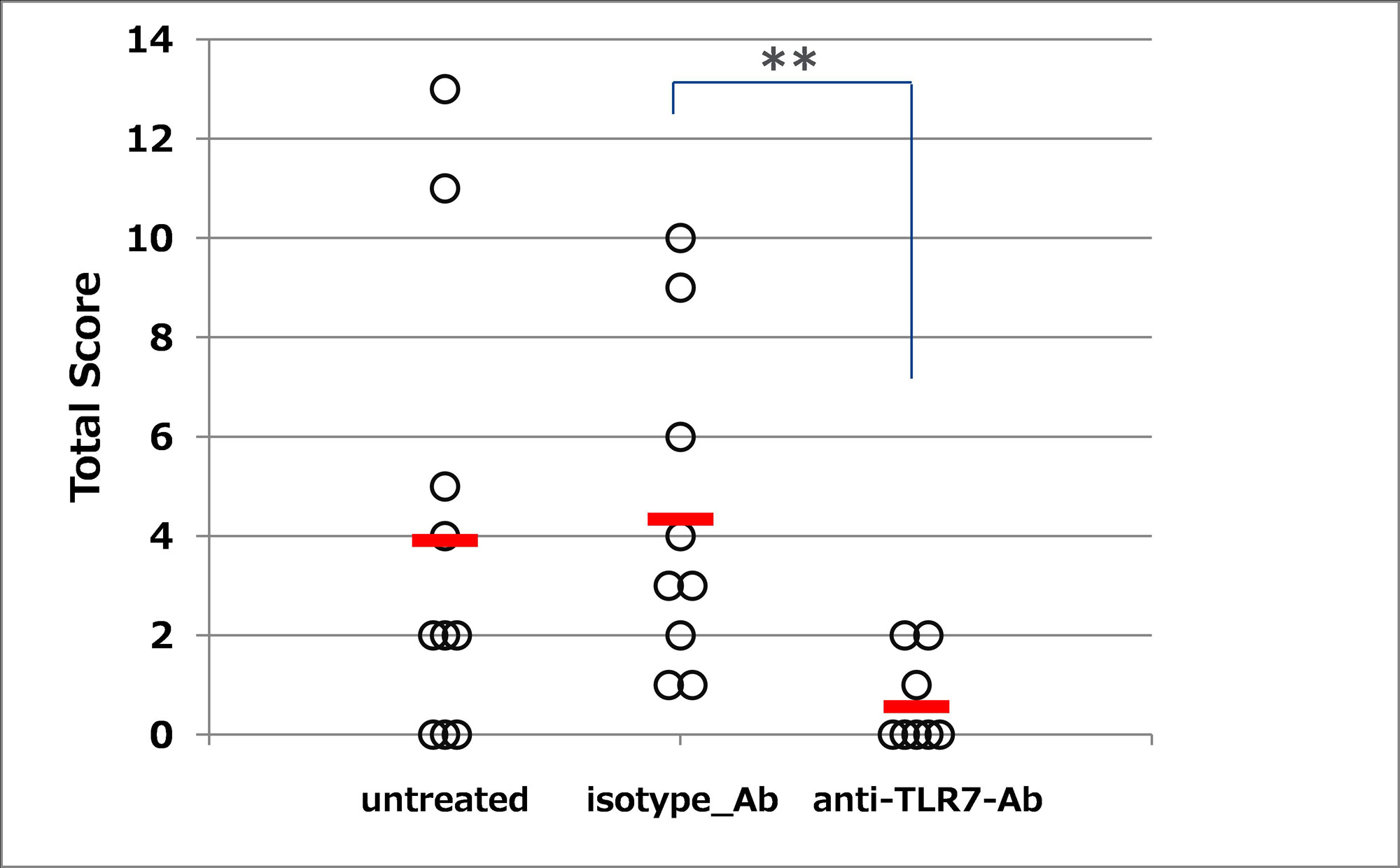
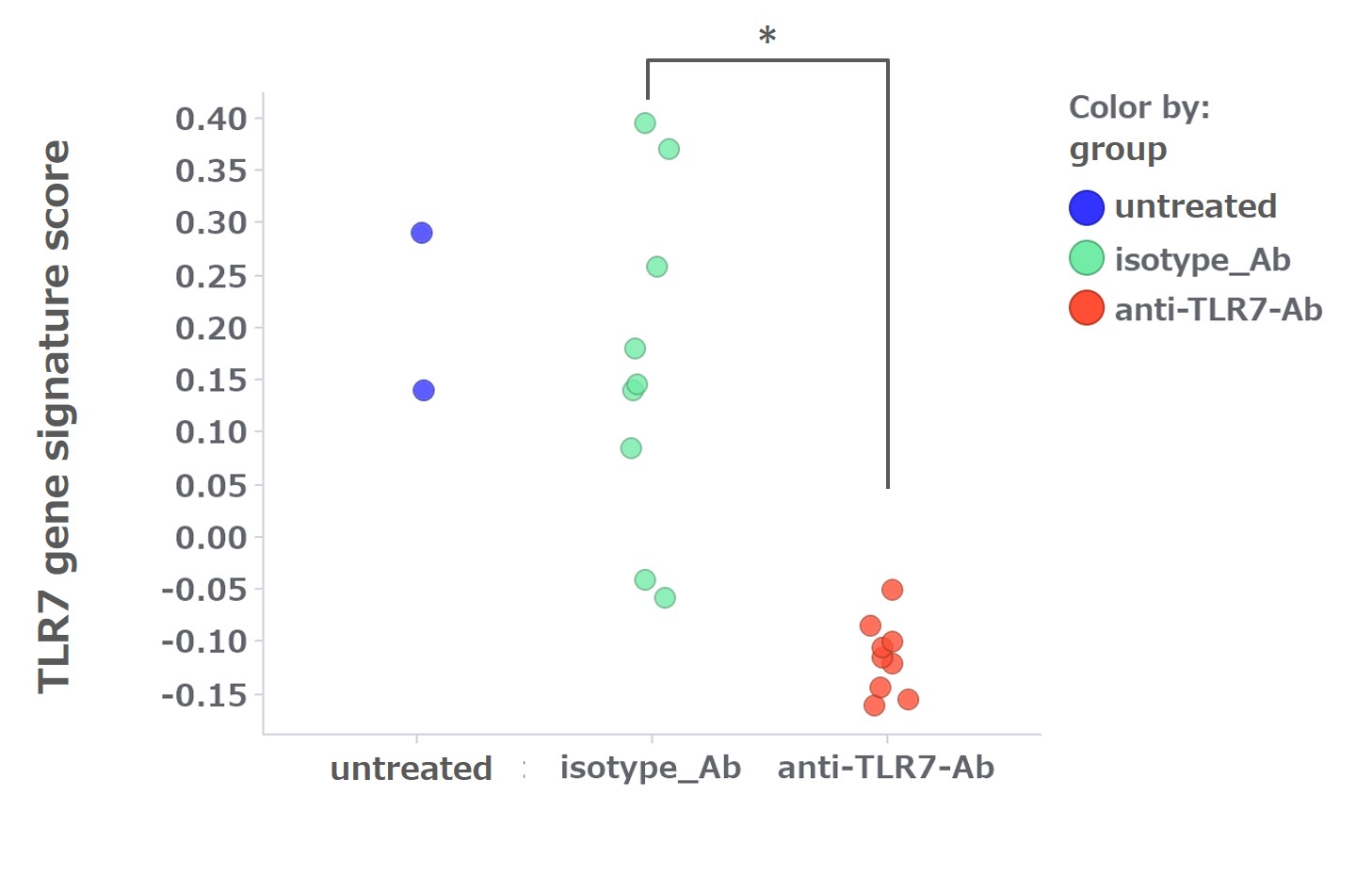
Results: DS-7011a bound to both human and cynomolgus monkey TLR7 but not to mouse or rat TLR7. DS-7011a bound to human TLR7 but not to other human TLR family proteins. DS-7011a suppressed the TLR7-stimulated production of IL-6 and IFN-alpha by human PBMCs. The surrogate anti-mouse TLR7 mAb suppressed the TLR7-stimulated IL-6 production by mouse PBMCs and it reduced kidney tissue damage (Figure 1), inhibited autoantibody production, and suppressed TLR7 GS expression in NZBWF1 mice (Figure 2). There were no toxicity findings in monkeys treated for 3 months at doses up to 400 mg/kg.
Conclusion: DS-7011a shows the ability to inhibit the TLR7-stimulated production of inflammatory cytokines and shows no toxicity in monkeys after 3-month treatment up to and including the maximum administered dose. An anti-mouse TLR7 mAb ameliorated manifestations of a mouse lupus model, suppressing the expression of TLR7 GS. These findings indicate that, by targeting TLR7, DS-7011a is a promising therapeutic option for the treatment of SLE.
To cite this abstract in AMA style:
Manno A, Honda T, Kuwata C, Ito S, Kadokura M, Mizutani R, Yamada S, Tomimori Y. Targeting Toll-Like Receptor 7 with DS-7011a, a Promising Novel Antagonistic Antibody for the Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/targeting-toll-like-receptor-7-with-ds-7011a-a-promising-novel-antagonistic-antibody-for-the-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-toll-like-receptor-7-with-ds-7011a-a-promising-novel-antagonistic-antibody-for-the-treatment-of-systemic-lupus-erythematosus/