Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Better understanding of epigenetic regulatory mechanisms in RA pathogenesis will facilitate the development of new biomarkers or therapeutic strategies. MicroRNAs are post-transcriptional regulators that co-ordinate cell activation by fine-tuning multiple intracellular pathways. We recently observed that microRNA-155 is up-regulated in RA B cells, particularly in anti-citrullinated protein antibodies (ACPA) positive RA patients. Herein, we examined the role of endogenous miR-155 in RA B cell function. Materials and
Methods: Peripheral blood (PB) was obtained from healthy controls (HC) and RA patients (2010 ACR/EULAR classification criteria). To asses endogenous levels of miR-155 in B cells subsets; CD19+ B cell from both groups (n=6 each) were stained with antibodies against na•ve and memory subsets specific markers (CD27, CD38, IgD and IgM) and sorted with FACSAriaIII followed by quantitative RT-PCR with miR-155 and U6 (housekeeping) specific primers. miR-155 in situ hybridisation combined with CD20 staining was performed on RA synovial tissues (n=5). CD19+ B cells of ACPA positive RA patients (n=5-6) were transfected with miR-155 inhibitors (miR-155I) or control inhibitor (CI). 48h later, quantitative RT-PCR was performed to identify transcription factors that regulate the B cell transcriptome and are under control of miR-155 (PAX5, PRDM1, PU.1 and IRF4). To evaluate antibody production (IgG and IgG-ACPA), CD19+ cells of ACPA+ RA patients (n=5) were transfected with miR-155 inhibitor or a control; and cultured in the presence of BAFF (20 ng/ml) and IL-6 (30 ng/ml) or were stimulated with a plate bound CD40 ligand (2 μg/ml), BAFF (100 ng/ml), IL-21 (50 ng/ml) and anti-IgM (5μg/ml). Each condition was performed in 14 replicates. Supernatants were assessed for the presence of total IgG and IgG-ACPA using standard or immune scan ELISA, respectively.
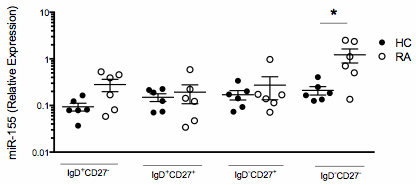
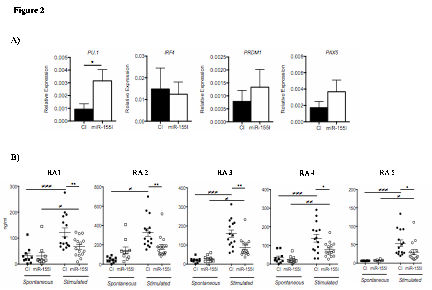
Results: Analysis of the expression of miR-155 in B cell subsets: na•ve, CD27–IgD+; pre-switched memory, CD27+IgD+; post-switched memory, CD27+IgD–; and double negative memory cells (CD27–IgD–) revealed that the double negative memory B cell population expressed the highest levels of miR-155, which was significantly higher in ACPA+RA compared to HC B cells (Figure 1). Furthermore, CD20 cells in ectopic follicular structures in RA synovium expressed high levels of miR-155. Functional studies revealed that inhibition of endogenous miR-155 in RA B cells led to the de-repression of PU.1 but did not impact PAX5, PRDM1 and IRF4 levels (Figure 2A). This was associated with the substantial reduction in BCR crosslinking induced antibody production by RA B cells (Figure 2B).
Conclusion: Our study demonstrates that antibody production by RA B cells is controlled by the miR-155-PU1 pathway. We propose that an inhibitor of miR-155 could be used to simultaneously treat both myeloid and lymphoid synovial phenotypes increasing the current response rate of RA patients.
To cite this abstract in AMA style:
Kurowska-Stolarska M, McInnes IB, Alivernini S, Elmesmari A, Ferraccioli G, Reilly J, McCarey DW. Targeting Mir-155 in Rheumatoid Arthritis B Cells Reduces Antibody Production [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/targeting-mir-155-in-rheumatoid-arthritis-b-cells-reduces-antibody-production/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-mir-155-in-rheumatoid-arthritis-b-cells-reduces-antibody-production/