Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Guidelines suggest glucocorticoids (GC) should be used as bridge therapy in rheumatoid arthritis (RA), but many patients are on chronic treatment, and the effects of withdrawal have not been studied extensively. The 2-year, double-blind GLORIA trial evaluated benefits and harms of low dose GC added to standard care (1). Senior RA patients (≥ 65 years) were randomly assigned to prednisolone 5 mg/day or placebo. For this study we examined disease activity, flares and signs of adrenal insufficiency after withdrawal of blinded trial medication.
Methods: After the final trial visit study medication was linearly tapered to zero in 3 months. Patients who successfully completed the trial and did not receive open-label GC during the 4 weeks after the final trial visit were included in this follow-up study.
The primary outcome was change in DAS28 at 3-month follow-up compared to the final trial visit. Secondary outcomes included the occurrence of disease flares (DAS28 increase > 0.6 or open-label GC between week 5 and 12 of the taper phase) and signs of adrenal insufficiency, assessed by 9 items selected from the 57-symptom list from the MDHAQ questionnaire and hypotension (systolic RR < 90 or diastolic RR < 60). In a subset of patients, cortisol and ACTH were measured in spot serum samples at the follow-up visit. Analysis of covariance and chi-square tests were used where appropriate, with one-sided testing for the primary outcome.
Results: 278 participants completed the GLORIA study, 21 received GC within 4 weeks after the end of the trial, 58 had missing data, leaving 199 patients. 34 patients received open label GC after 4 weeks and were excluded for the primary analysis. In the remaining 165 patients (80 prednisolone, 85 placebo), mean (SD) DAS28 was higher on placebo: 3.14 (1.04) vs 2.92 (1.13) prednisolone at the final trial visit. After tapering, disease activity increased significantly (p=0.02) in the prednisolone group to 3.18 (1.20) but was stable in placebo (3.14). The difference in the increase of DAS28 between the groups was 0.21 (95%CL –0.01, p=0.06). 44 of 99 prednisolone patients flared on tapering vs 31 of 100 placebo, relative risk 1.43 (95%CI 0.99; 2.07; p=0.07).
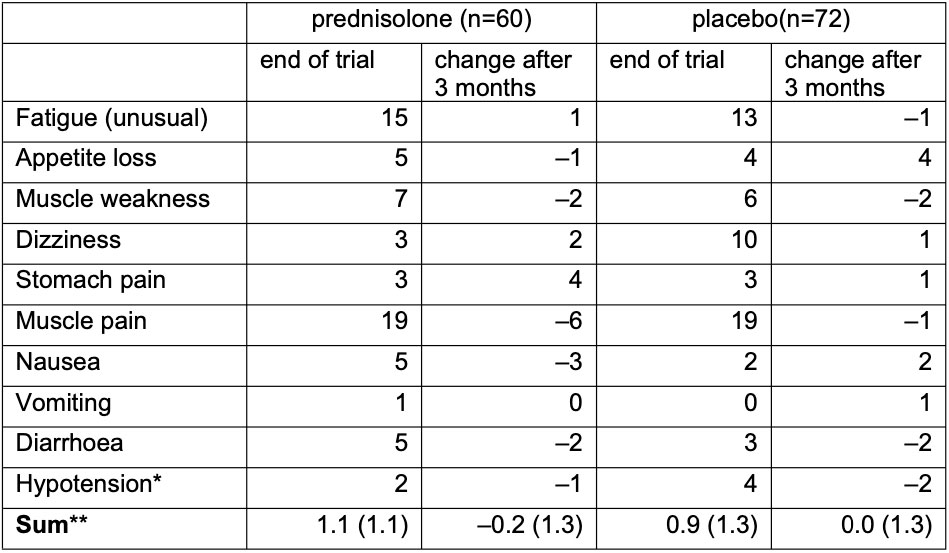
For signs of adrenal insufficiency, 60 prednisolone and 72 placebo patients were available (Table 1). Mean (SD) number of signs for prednisolone was 1.1 (1.1) versus 0.9 (1.3) for placebo at final trial visit and 0.8 (1.2) versus 0.8 (1.0) at follow-up. Difference in the change of the number of signs was –0.1 (95%CI –0.4;0.3; p=0.66). No differences were seen in ACTH or cortisol levels: mean (SD) ACTH was 5.8 (4.1) in 23 prednisolone patients, and 5.1 (3.7) in 24 placebo patients; cortisol 310 (166) v 296 (113), cortisol/ACTH 67 (40) v 77 (54). Two prednisolone and one placebo patient had cortisol levels below 83. None developed clinical hypoadrenalism during further follow-up.
Conclusion: Tapering prednisolone moderately increases disease activity to placebo levels (mean still at low disease activity levels) and numerically increases the risk of flare without any evidence of adrenal insufficiency. This suggests that withdrawal of low dose prednisolone is feasible after 2 years of administration.
1. Boers M. ARD 2022; doi:10.1136/annrheumdis-2021-221957
** Mean (SD)
To cite this abstract in AMA style:
Almayali A, Boers M, Hartman L, OPRIS-BELINSKI D, Bos R, Kok M, Pereira da Silva J, Griep E, Klaasen R, Allaart C, Baudoin P, Raterman H, Szekanecz Z, Buttgereit F, MASARYK P, Lems W, Cutolo M, ter Wee M. Tapering of Long-term, Low Dose Glucocorticoids in Senior Rheumatoid Arthritis Patients: Follow up of the Pragmatic, Multicentre, Placebo-controlled GLORIA Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/tapering-of-long-term-low-dose-glucocorticoids-in-senior-rheumatoid-arthritis-patients-follow-up-of-the-pragmatic-multicentre-placebo-controlled-gloria-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tapering-of-long-term-low-dose-glucocorticoids-in-senior-rheumatoid-arthritis-patients-follow-up-of-the-pragmatic-multicentre-placebo-controlled-gloria-trial/