Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: To determine whether prednisolone (PSL) could be tapered or discontinued without deterioration of disease control through optimizing methotrexate (MTX) for 2-yrs in patients with rheumatoid arthritis (RA) under stable treatment.
Methods: Patients with RA who fulfilled ARA 1987 criteria and/or ACR/EULAR 2010 criteria and had regularly visited our clinic for ≥1year under stable treatment were consecutively registered during Sep-Oct 2016. Clinical demographics as well as disease status and medication at baseline (BL), year-1- and -2 were collected. The basic therapeutic strategy was to increase MTX while reducing PSL as much as possible, based on patient’s consent agreement. Initiation of biological DMARDs (bDMARDs) or JAK inhibitors (JAKi) was allowed if required.
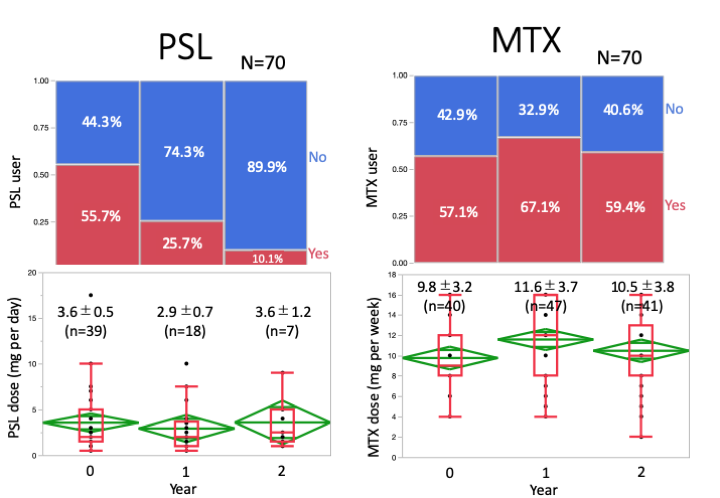
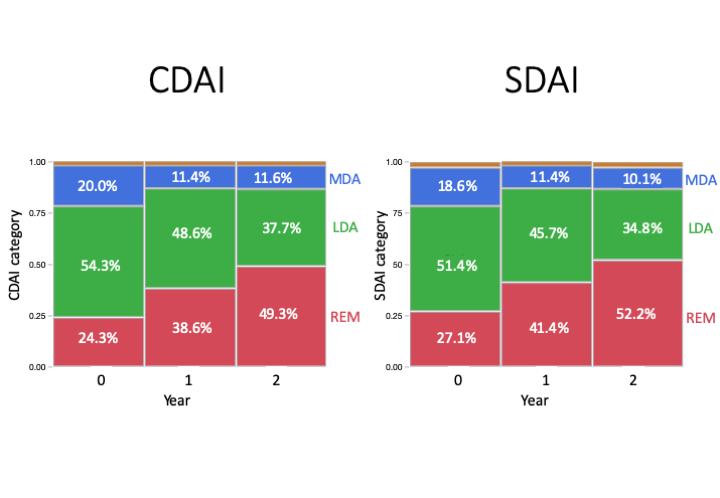
Results: 70 patients were enrolled to the study. Age (median [IQR]) 62 [51, 68] years; female 69%; disease duration 6.8 [3.4, 13.7] years. Proportion of the patients using MTX at BL, 1 and 2 years were 57, 67 and 59%, respectively, while doses (mean ± SD) of MTX were 9.8 ± 3.2, 11.6 ± 3.7 and 10.5 ± 3.8 mg per week, respectively. Percentage of the patients using PSL were 56, 26 and 10%, respectively, while doses of PSL were 3.6 ± 3.4, 2.9 ± 2.6 and 3.6 ± 2.8mg per day, respectively. bDMARDs/JAKi users were 16, 18 and 26, newly initiated in 2 and 8 during BL-1-2years. CDAI / SDAI / DAS28(ESR) -remission ratio at BL, 1-year and 2-years were; 24/ 39/ 49%, 27/ 41/ 52%, and 35/ 41/ 48%, respectively. These results were consistent in patients without bDMARDs/JAKi. Severe adverse events were found in two patients (peritoneal cancer in one, myelodysplastic syndromes in one).
Conclusion: PSL was successfully discontinued in 90% of the patients by optimizing MTX with or without bDMARDs/JAKi with improved disease control in patients with RA under stable treatment. However, tapering PSL to withdrawal required more than 6 months in the majority of the patients that might delay clinical decision-making of starting bDMARDs or JAKi.
To cite this abstract in AMA style:
Hirata S, Omoto T, Kohno H, Watanabe H, Yukawa K, Tokunaga T, Kuranobu T, Oi K, Yoshida Y, Sugimoto T, Mokuda S, Oda K, Nojima T, Sugiyama E. Tapering and Discontinuing Prednisolone Without Deteriorated Disease Control by Optimizing Methotrexate in Patients with Rheumatoid Arthritis Under Stable Treatment – 2-year Results in the Real-world Clinical Practice – [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/tapering-and-discontinuing-prednisolone-without-deteriorated-disease-control-by-optimizing-methotrexate-in-patients-with-rheumatoid-arthritis-under-stable-treatment-2-year-results-in-the-rea/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tapering-and-discontinuing-prednisolone-without-deteriorated-disease-control-by-optimizing-methotrexate-in-patients-with-rheumatoid-arthritis-under-stable-treatment-2-year-results-in-the-rea/