Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Vaccination against SARS-CoV-2 is particularly important for patients with systemic lupus erythematosus (SLE), who may be at increased risk of hospitalization for COVID-19. However, the most common reason for vaccine refusal in patients with SLE is fear of SLE disease flare. Additionally, the SARS-CoV-2 mRNA vaccines could potentially induce interferon production, associated with increased SLE disease activity. Thus far, no population-based data exist regarding whether SARS-CoV-2 vaccines trigger SLE flares.
Methods: We emailed a secure web-based survey on March 5, 2021 to 7,094 patients aged ≥18 years evaluated at least once between 2018-2020 by a rheumatologist at a single Rheumatology Division in New York City. We included individuals who received at least one vaccine dose. ICD-10-CM codes identified patients with SLE. A self-reported disease flare was defined as “a sudden worsening of your rheumatology condition or arthritis” within two weeks of the vaccine dose. To prevent misleading conclusions, we did not perform statistical testing on these interim results.
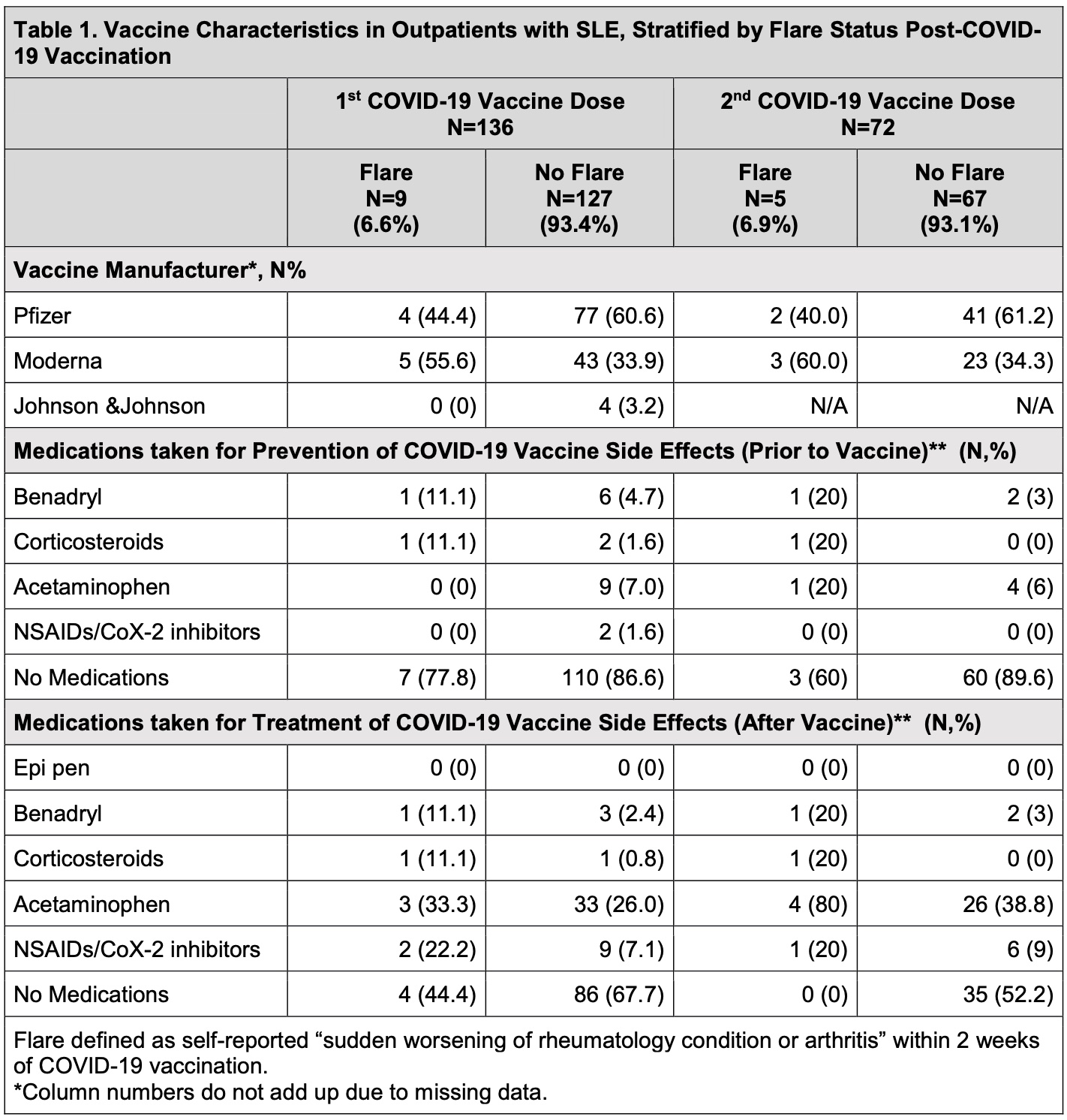
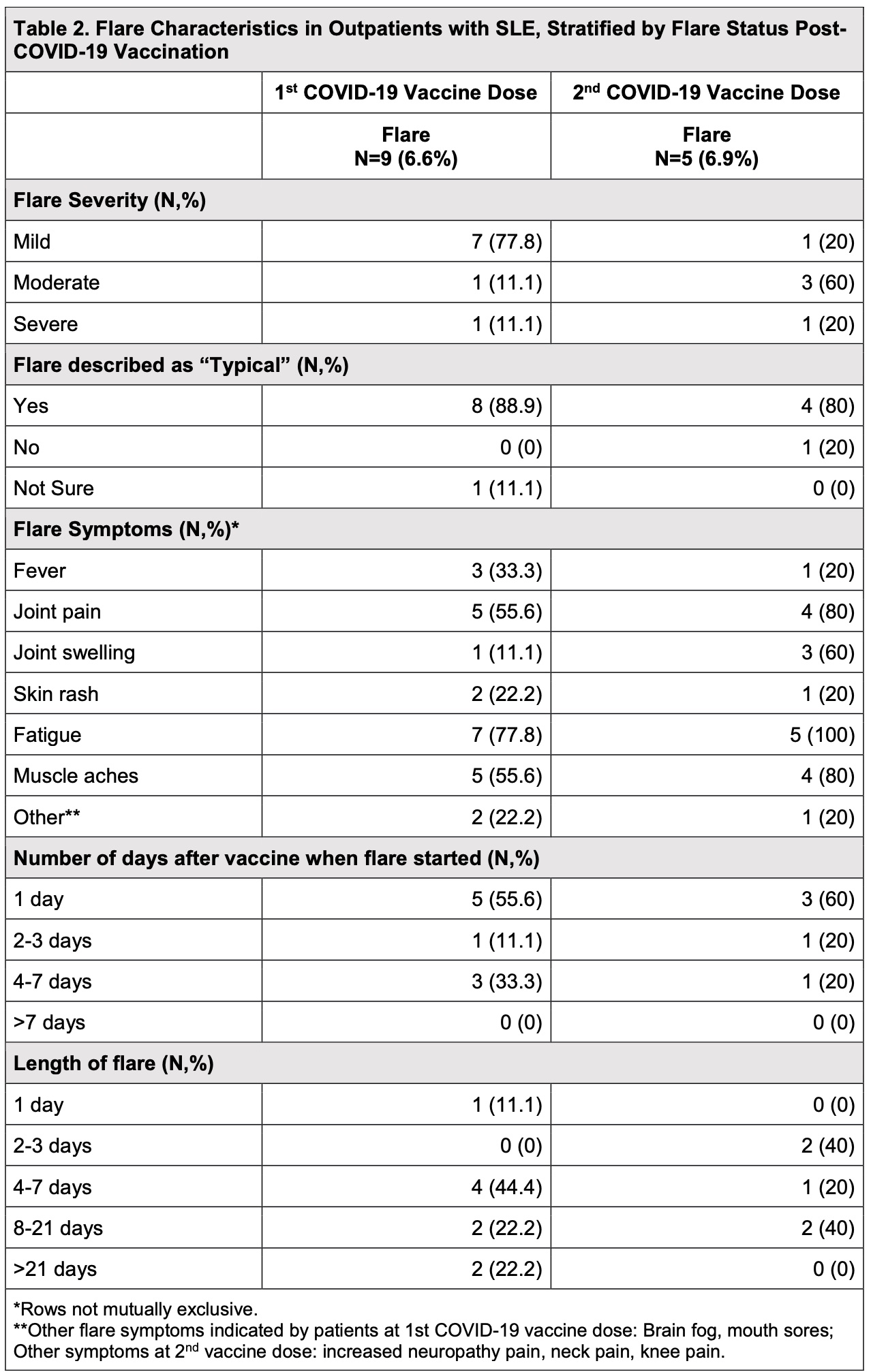
Results: As of March 30, 2021, 136/466 (29.2%) respondents with SLE (mean[SD] age 54.7 [13.9] years; 93.4% female; 67.7% White; 13.2% Hispanic/Latinx ethnicity) reported receiving at least one COVID-19 vaccine dose. Eighty-one patients (59.6%) received Pfizer, 48 (39.3%) received Moderna, and 4 (2.9%) received Janssen. Of patients receiving Pfizer or Moderna, 72 (54.5%) received 2/2 doses. Twelve patients (8.8%) reported SLE flare within two weeks of COVID-19 vaccination. Only 1 patient (8.3%) with flare reported a history of suspected/confirmed COVID-19, similar to those without flare (8.4%). Patients reporting SLE flare were older (59.0 [14.0] vs. 54.3 [13.9] years) and White (83.3% vs. 61.1%). Flares occurred in 12.5% of patients receiving Moderna and 7.4% receiving Pfizer (6 patients each). Out of 7 patients receiving both vaccine doses, 2 flared only after the first dose, 3 flared only after the second dose, and 2 flared after both doses. Of the 14 flare episodes, 9 occurred after the first dose, and 5 occurred after the second dose. Medications were used for prevention or treatment of vaccine side effects by both the flare and non-flare groups (Table 1). Most flares after the first vaccine dose were mild (77.8%), and after the second vaccine were moderate (60%). 12/14 flares (85%) were described as “typical”, predominantly characterized by joint pain, muscle aches, and fatigue (Table 2). While 8/14 flares started 1 day after vaccination, 4/14 started 4-7 days later. None started >7 days post-vaccination. Most SLE flares resolved within 7 days of onset; however, 4/14 lasted 8-21 days and 2/14 lasted >21 days.
Conclusion: Our data suggest >91% of SLE patients did not experience a disease flare post-SARS-CoV-2 vaccination; of those that did, most had mild flares. Given almost all patients reported that their post-vaccine flare was “typical” of their SLE flares, vaccine side effects alone may not explain these findings. This information can help inform vaccine decision-making among patients with SLE. Future analyses will evaluate whether modifying immunosuppressive medications to enhance vaccine efficacy increased SLE flare risk.
To cite this abstract in AMA style:
Barbhaiya M, Levine J, Siegel C, Bykerk V, Jannat-Khah D, Mandl L. Systemic Lupus Erythematosus Disease Flares After SARS-CoV-2 Vaccination [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/systemic-lupus-erythematosus-disease-flares-after-sars-cov-2-vaccination/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systemic-lupus-erythematosus-disease-flares-after-sars-cov-2-vaccination/