Session Information
Date: Saturday, November 16, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Recent evidence suggests lower response rates to tumor necrosis factor inhibitors (TNFi) among women with non-radiographic axial spondyloarthritis (nr-axSpA). However, there is a paucity of studies exploring the interaction between axSpA subtype and sex on first-line biologic discontinuation, which is a composite measure of drug performance in the real-world setting. We aimed to assess the relationship of subtype and sex with first-biologic discontinuance in axSpA patients and to determine whether these factors interact to affect treatment retention.
Methods: We studied 469 axSpA patients followed longitudinally with start dates for their first TNFi or interleukin-17 inhibitor (IL-17i). All patients fulfilled the ASAS classification criteria and were further subdivided into 4 groups according to subtype and sex. Biologic survival rates were compared by subtype, sex, and the 4 patient groups. We used multiple imputation by chained equations to address missing data. Modified Poisson regressions were performed to calculate risk ratios (RR) and 95% CI for the association of biologic discontinuation with subtype, sex, and their interaction. The interaction between subtype and sex was further assessed by estimating the relative excess risk due to interaction (RERI) and ratio of risk ratios (RRR). The magnitude of the effect of an unmeasured confounder needed to explain the association between the exposures and the outcome was estimated using E-values.
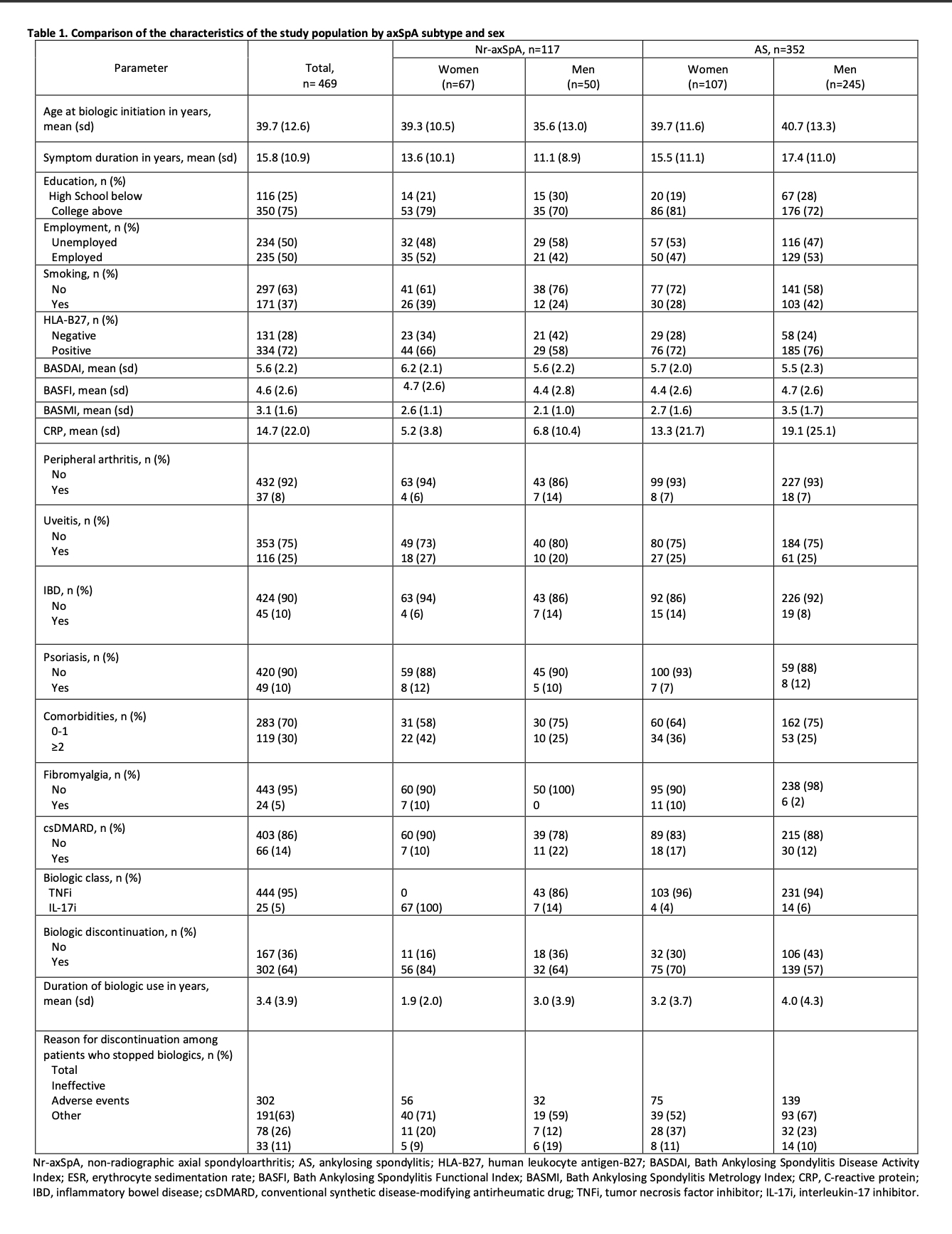
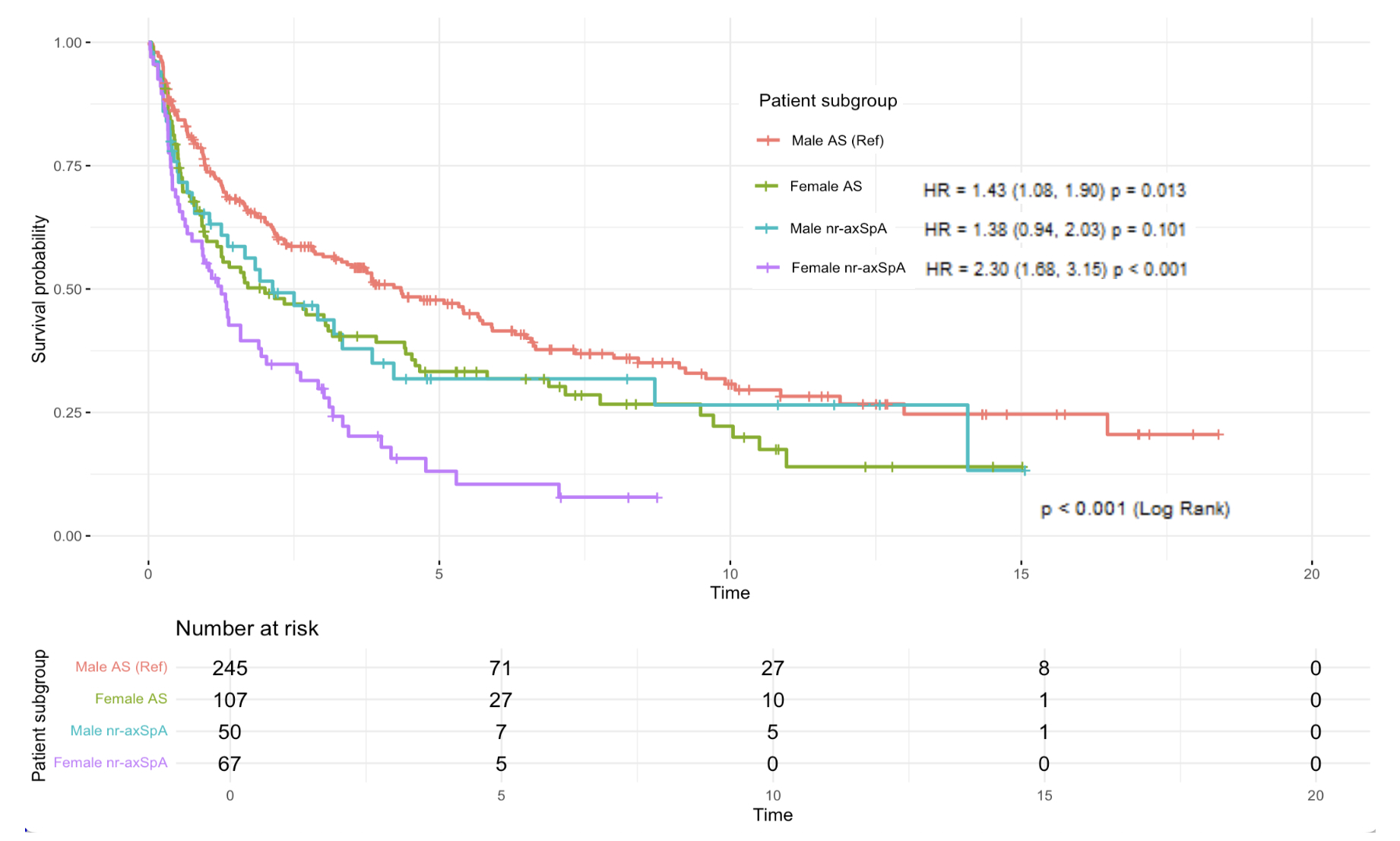
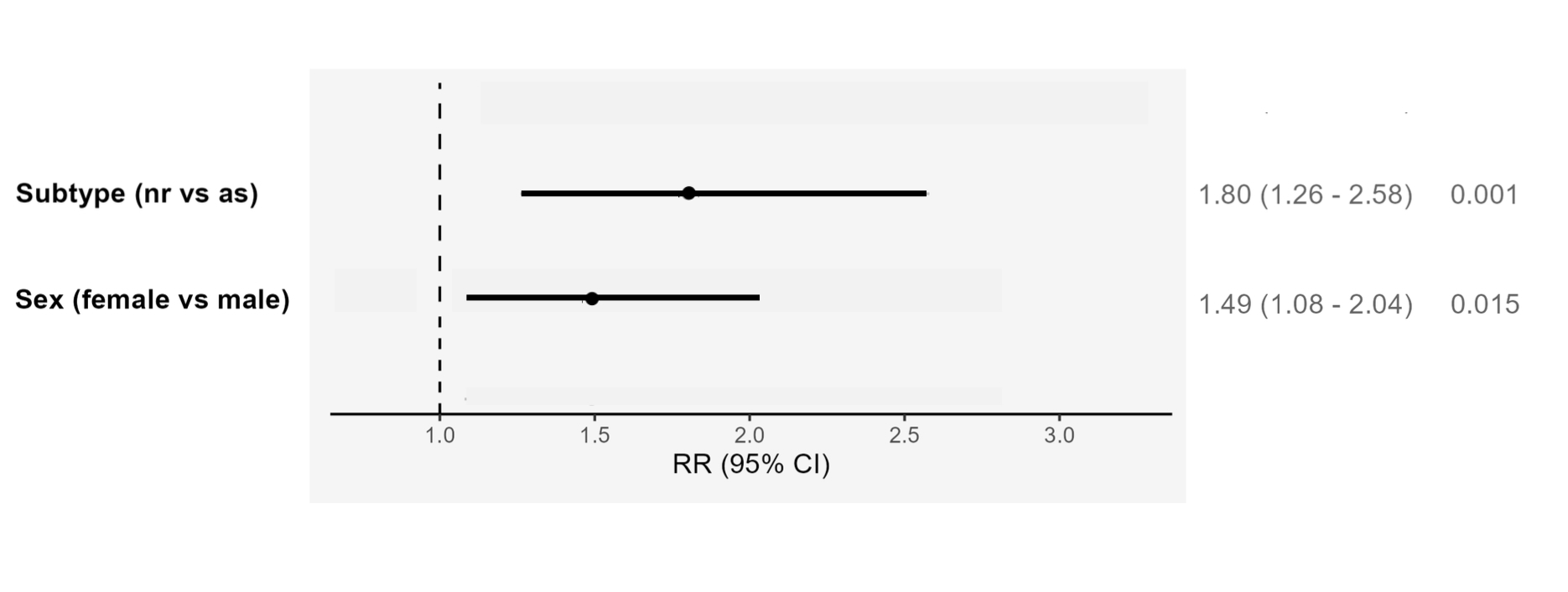
Results: Our cohort consisted of mostly male patients (63%) with radiographic axSpA (r-axSpA) (75%). A total of 302 patients (64%) discontinued their first biologic during follow-up. Female nr-axSpA patients at baseline had the lowest BASDAI and highest mean CRP, and most had ≥2 comorbidities (Table 1). Biologic survival over 20 years was significantly lower in nr-axSpA females (vs r-axSpA males) patients at all time points. Compared with r-axSpA males, discontinuation risk was twice as high in nr-axSpA females (HR 2.30, 95% CI 1.68 to 3.15, p < 0.001), mostly due to secondary nonresponse to biologics (Figure 1). Nr-axSpA (RR 1.80, 95% CI 1.26 to 2.59, p=0.001) and female sex (RR 1.49, 95% CI 1.081 to 2.045, p=0.015) were independently associated with biologic discontinuation (Figure 2). Jointly, nr-axSpA and female sex increased the risk of stoppage (RR 2.70, 95% CI 1.73 to 4.20, p< 0.001). The high E-values (3.01 for subtype, 2.34 for sex) indicated robustness of our effect estimates to residual confounding. Positive interaction trends between subtype and sex were seen on both the additive (RERI 0.49, 95% CI -0.78 to 1.75) and multiplicative (RRR 1.05, 95% CI 0.55 to 2.03) scales.
Conclusion: The nr-axSpA subtype and female sex were associated with shorter biologic survival times over a 20-year period and a significantly increased risk of first-line biologic discontinuance. There was evidence suggesting that the risk of drug termination was higher when both factors were present than when each factor was considered individually. The overall reduced biologic survival places nr-axSpA women at higher risk of poorer treatment outcomes.
To cite this abstract in AMA style:
Remalante-Rayco P, Baja E, Baskurt Z, Chim T, Inman R, Dans L, Haroon N. Synergistic Impact of the Non-radiographic Axial Spondyloarthritis Subtype and Female Sex on First-Line Biologic Discontinuation : A Study of Interaction Effects [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/synergistic-impact-of-the-non-radiographic-axial-spondyloarthritis-subtype-and-female-sex-on-first-line-biologic-discontinuation-a-study-of-interaction-effects/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/synergistic-impact-of-the-non-radiographic-axial-spondyloarthritis-subtype-and-female-sex-on-first-line-biologic-discontinuation-a-study-of-interaction-effects/