Session Information
Date: Tuesday, November 12, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Patient Preferences, Beliefs, & Experiences
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic inflammatory disease resulting in significant symptom burden.1 Over time and without adequate treatment, PsA can lead to disability and reduced patient quality of life.2 Fatigue is among the most common symptoms,1 but is complex and difficult to measure. The most burdensome symptoms that impact PsA patients need to be better understood in order to select patient reported outcomes (PRO) tools that adequately capture these concepts.
Objectives:
- Identify the signs and symptoms of PsA experienced by patients, with a focus on fatigue, and how the disease and its treatment(s) impact patients’ lives.
- Assess the content validity of select PRO instruments and items to measure fatigue.
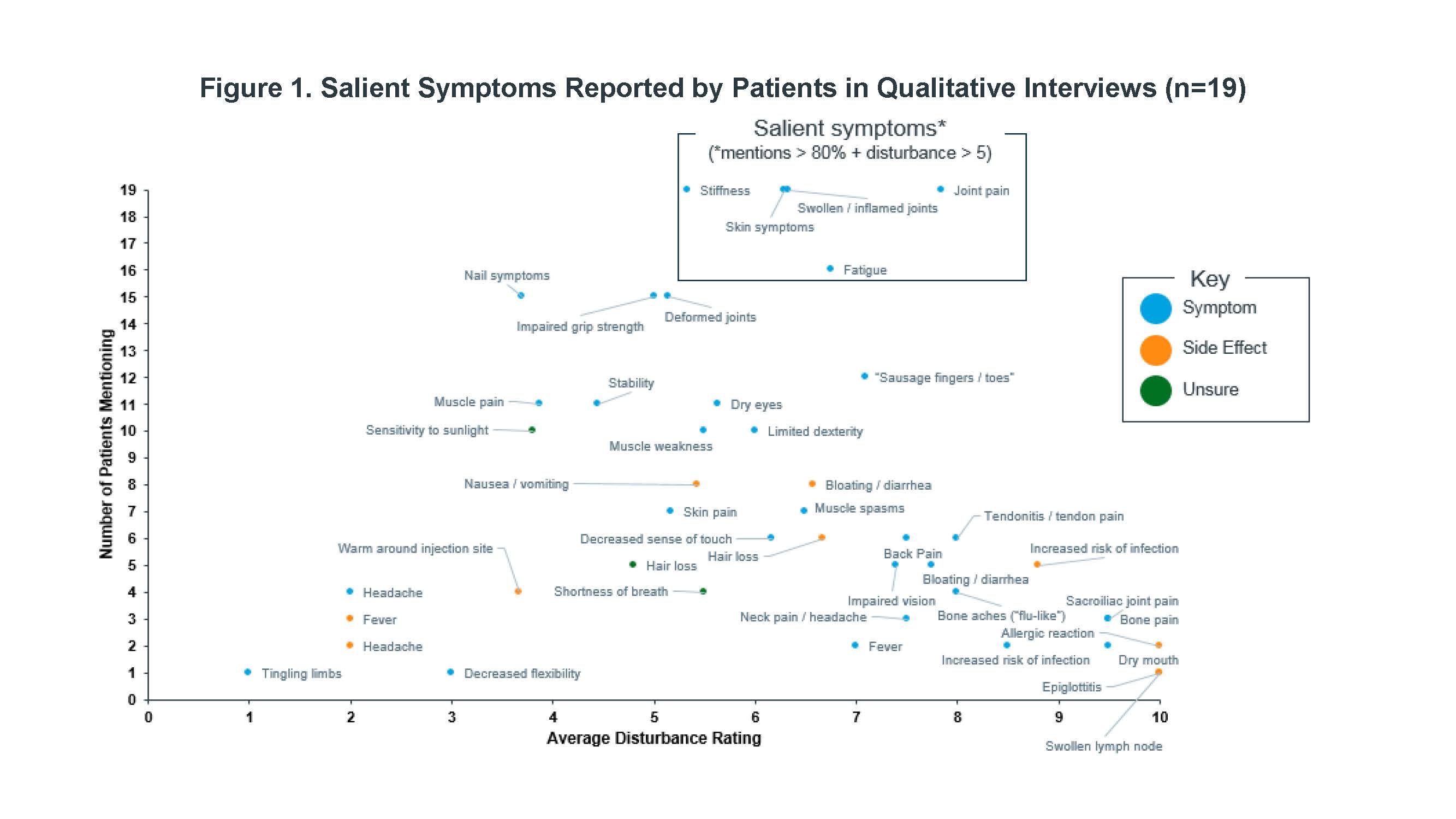
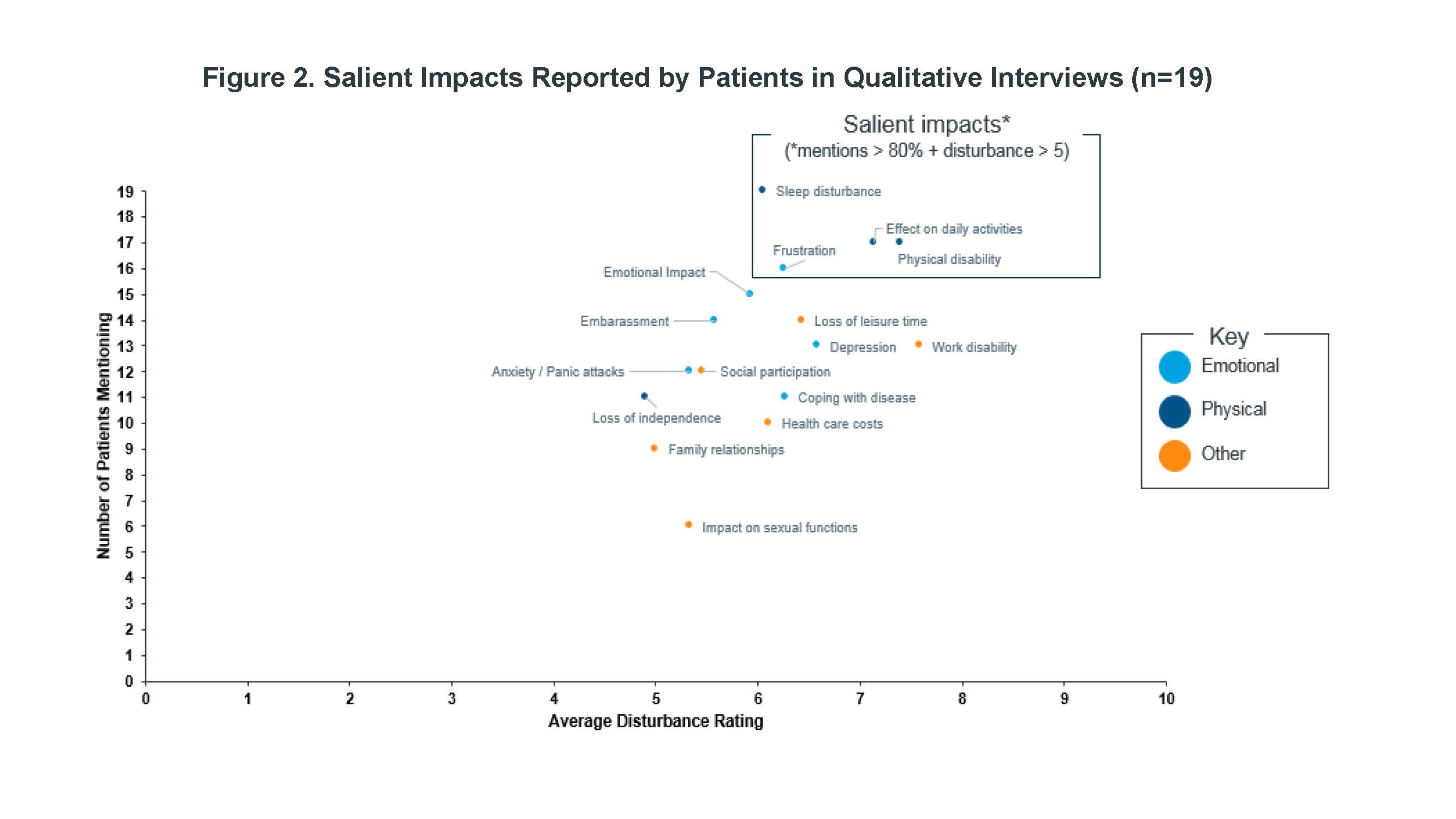
Methods: Qualitative interviews were conducted among patients with PsA recruited through the FORWARD databank, all of which satisfy the ACR classification for PsA. The most frequently experienced symptoms and impacts of PsA and the degree to which they disturbed patients’ lives, were tabulated. Disturbance was evaluated on a scale from 0 (not at all) to 10 (greatly disturbs). Patients reporting fatigue were probed to describe the experience in their own words, and descriptors were recorded. Interviews were conducted and assessed on a rolling basis and recruitment continued until concept saturation was achieved.
Results: Nineteen PsA patients were interviewed for this study. A core set of PsA symptoms were identified by nearly all patients and with moderate to high average disturbance ratings (Figure 1): joint pain, skin symptoms, stiffness, swollen/inflamed joints, and fatigue. The most salient impacts (Figure 2) were sleep disturbance, physical disability, effects on daily activities, and feelings of frustration. Most common descriptors of fatigue included “fatigue,” “tiredness,” “lack of energy,” “mental fatigue,” and “exhaustion.”
Conclusion: Salient symptoms were consistent with those previously reported, along with a broader range of symptoms and impacts, which included fatigue. In addition to physical disability, others such as sleep disturbance, frustration, and effect of daily activities were common high impact themes that emerged.
To cite this abstract in AMA style:
Michaud K, Alemao E, Nowak M, Bruce R, Cantor S, Hintzen C, Mease P, DeBusk K, Ogdie A. Symptoms and Impacts in Psoriatic Arthritis: Findings from Qualitative Patient Interviews [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/symptoms-and-impacts-in-psoriatic-arthritis-findings-from-qualitative-patient-interviews/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/symptoms-and-impacts-in-psoriatic-arthritis-findings-from-qualitative-patient-interviews/