Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: TNF inhibitors (TNFi) are currently the most commonly used first-line targeted therapy for patients with rheumatoid arthritis (RA). After discontinuation of the first TNFi, another TNFi may be prescribed, or the patient may be switched to a biologic agent with another mode of action (OMA) or to a Janus kinase inhibitor (JAKi). Current ACR guidelines conditionally recommend switching to OMA or JAKi after discontinuation of the first TNFi (1), however supporting evidence is limited. This study aimed to evaluate the survival on treatment with second-line targeted therapy in RA patients after discontinuing the first TNFi using data from the Czech national registry ATTRA.
Methods: Adult patients with RA who had started second-line targeted therapy with second TNFi, OMA, or JAKi after discontinuation of the first TNFi from 1 January 2019 to 1 April 2024 were included. Baseline patient characteristics at initiation of the second-line targeted therapy were compared and yearly survival rates and median survival times for all three groups were calculated using raw data. In the second step, propensity score was employed to match patients based on gender, age, smoking status, RA duration, RA activity, concomitant treatment and presence of comorbidities, after which survival between TNFi and OMA + JAKi combined was compared. Finally, comparison of survival between individual groups (i.e. TNFi vs OMA, TNFi vs JAKi and OMA vs JAKi) in propensity score matched patients was performed.
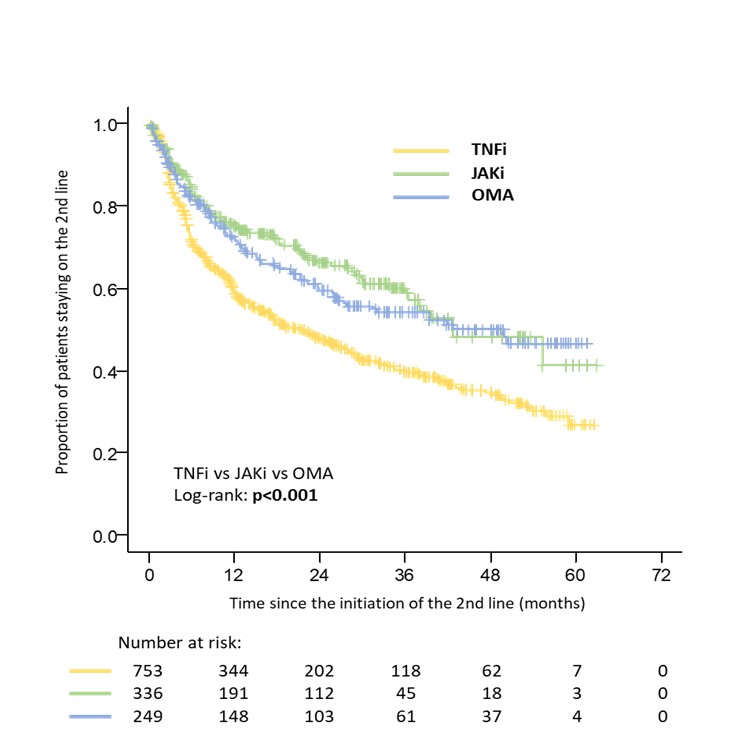
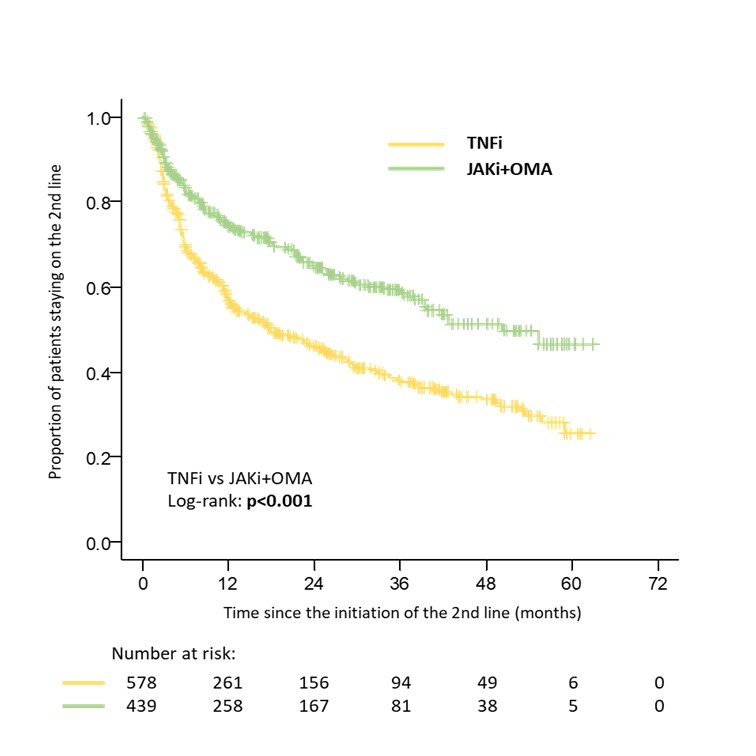
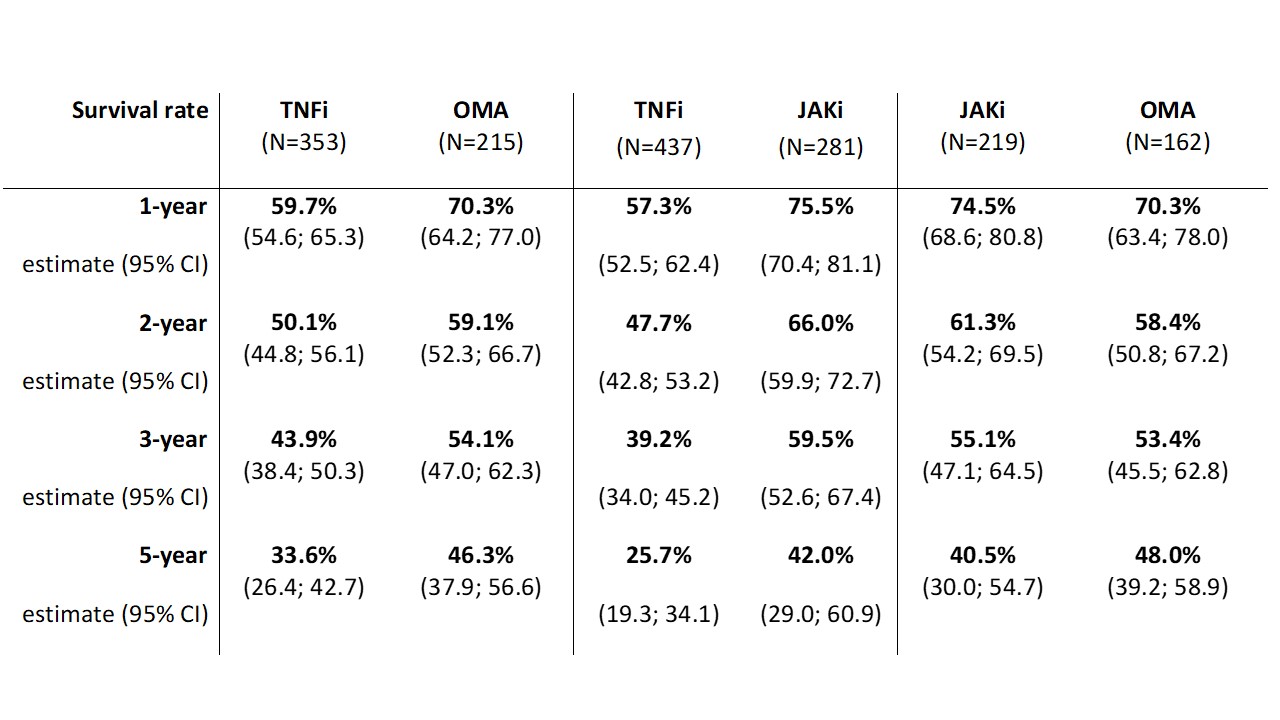
Results: Data from 1338 patients fulfilling inclusion criteria were available for initial analysis. 753 patients started second TNFi, 249 started OMA and 336 started JAKi after discontinuation of the first TNFi. The crude overall drug retention was higher in patients switching to OMA or JAKi compared to second TNFi with a median survival of 42.8 (31.1; 54.5), 42.8 (NA) and 21.1 (16.2; 26.0) months respectively (Figure 1). After propensity score matching the median treatment survival was 50.3 months (NA) in a combined group of 439 patients who switched to OMA or JAKi compared to 17.8 months (12.3; 23.3) in 578 patients on second TNFi (Figure 2). There was a significant difference in drug retention favoring OMA over second TNFi and JAKi over second TNFi in direct comparison, with no significant difference between OMA and JAKi (Table 1).
Conclusion: Among RA patients after discontinuation of the first TNFi, switching to OMA or JAKi is associated with significantly better drug survival compared to a second TNFi.
Acknowledgement: This study was supported by the project of MHCR for conceptual development of research organization 00023728
1) Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73(7):1108-1123.
To cite this abstract in AMA style:
Mann H, Baranová J, Horak P, Pavelka K, Šenolt L, Vencovský J, Kusalová K, Závada J. Switching to a Targeted Drug with a Different Mode of Action After Discontinuation of the First TNF Inhibitor Is Associated with Better Drug Survival Compared to a Second TNF Inhibitor in Rheumatoid Arthritis: A Propensity Score-matched Analysis from the Czech ATTRA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/switching-to-a-targeted-drug-with-a-different-mode-of-action-after-discontinuation-of-the-first-tnf-inhibitor-is-associated-with-better-drug-survival-compared-to-a-second-tnf-inhibitor-in-rheumatoid-a/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/switching-to-a-targeted-drug-with-a-different-mode-of-action-after-discontinuation-of-the-first-tnf-inhibitor-is-associated-with-better-drug-survival-compared-to-a-second-tnf-inhibitor-in-rheumatoid-a/