Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: A subset of patients with rheumatoid arthritis (RA) experience refractory disease and do not attain remission imparting worse long-term outcomes. We evaluated RA patients with at least one year of follow-up on their first biologic, to identify characteristics associated with multiple failures of advanced therapies (MFAT) (biologics and/or JAK inhibitors).
Methods: The Rheumatoid Arthritis Pharmacovigilance Program and Outcomes Research in Therapeutics (RAPPORT) registry is a prospective inception cohort of northern Albertan RA patients starting their first biologic. We assessed RAPPORT patients with at least one year of follow-up on a biologic and identified RA patients with MFAT (defined as those exposed to 3 or more biologic classes or JAK inhibitors) developing over follow-up (i.e. forward from our time zero, which was from one year after biologic initiation). Cox regression analysis was performed to evaluate factors (demographics, clinical characteristics, and treatment) potentially associated with poor outcome.
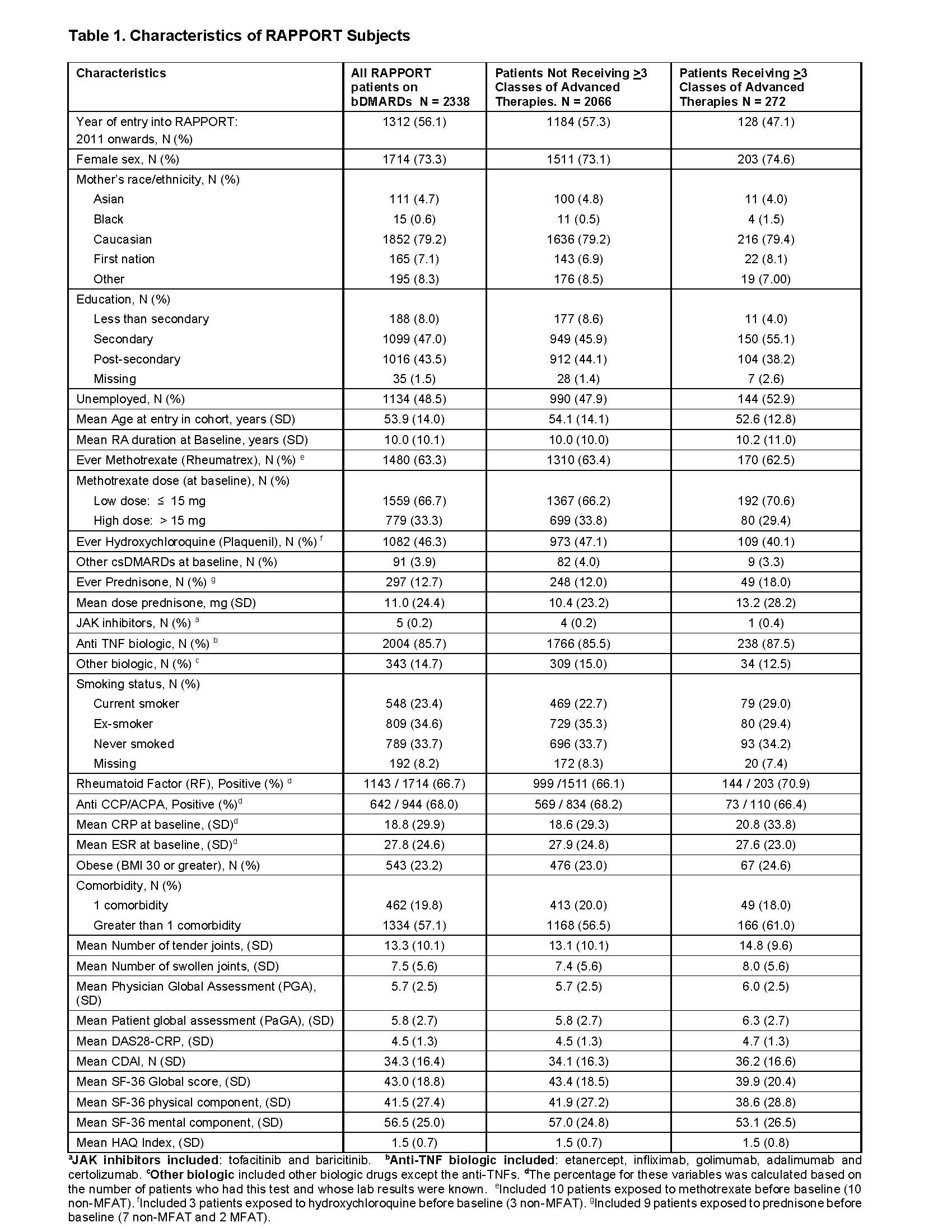
Results: Of 2338 RAPPORT patients, baseline characteristics included low exposure to prednisone (12.7%), mean DAS-28-CRP 4.5 (SD 1.3), mean HAQ 1.5 (SD 0.7) and 86% with anti-TNF as their first biologic. 2107 had at least one year of follow-up on their first biologic (231 dropped out or were lost to follow-up prior to this point). Over an average of 6 years of follow-up beyond our time zero, 271/2107 (13%) of patients ultimately required ≥3 advanced therapies. Characteristics were similar between the two groups concerning age, sex, RA duration, Caucasian maternal race, body mass index, seropositivity for rheumatoid factor and anti-CCP (Table 1). Current smoker (29%) and unemployed (53%) were characteristics observed more frequently in the MFAT group. In univariate analyses, high disease activity and lower education were associated with requiring ≥3 advanced therapies, and lower risk of the outcome was seen in those with DAS28 remission at 3-4 months (HR 0.49, 95% CI 0.35- 0.68).
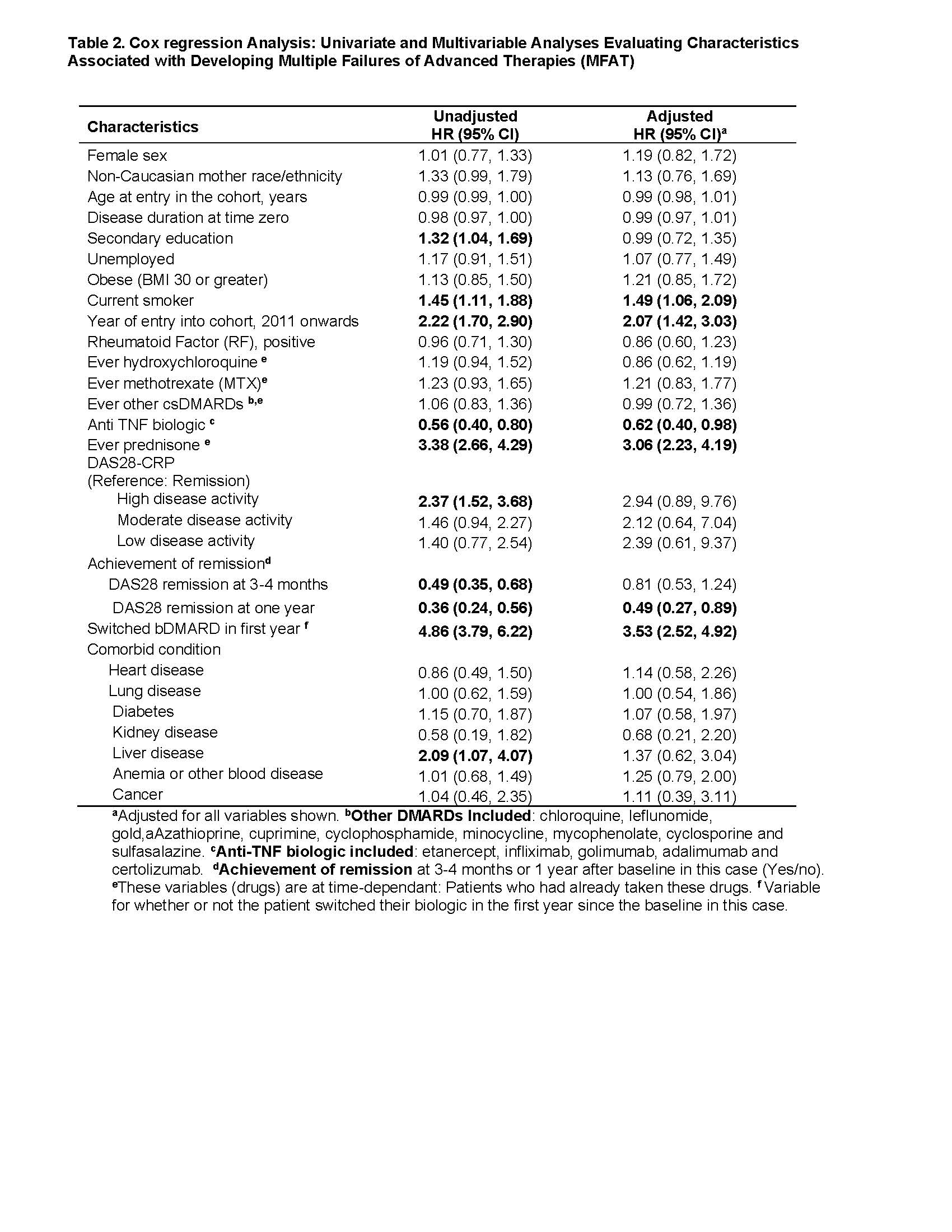
In unadjusted and adjusted models (Table 2), the risk of developing MFAT was associated with current smoking (time zero) (HR 1.49, 95% CI 1.06-2.09], entry into the cohort from 2011 onwards (HR 2.07, 95% CI 1.42-3.03], ever (up to time zero) used prednisone (HR 3.06, 95% CI 2.23-4.19]; and patients who switch bDMARD in the first year (HR 3.53, 95% CI 2.52-4.92). Lower risk of the outcome was associated with anti-TNF used as first biologic (HR 0.62 , 95% CI 0.40-0.98) and DAS28 remission at one year (HR 0.49, 95% CI 0.27-0.89). Results were similar when patients switching biologic in the first year were excluded. A potential limitation related to reasons for therapy changes beyond uncontrolled RA (e.g. adverse events).
Conclusion: Attaining disease remission within the first year of biologic initiation is a key factor associated with better longer-term RA outcomes. The greater risk of MFAT in early switchers may reflect the importance of personalized medicine and using the right treatment in the right patient in a timely manner. Subjects entering RAPPORT in the past decade were more likely to ultimately require ≥3 advanced therapies, possibly representing increasing therapy options and/or more aggressive approaches.
To cite this abstract in AMA style:
Keeling S, Jones B, Hall J, Homik J, Russell A, Lukusa L, Bernatsky S, Maksymowych W. Switching Biologics and Failure to Attain Remission in the First Year Predicts bDMARD Refractory Disease in Rheumatoid Arthritis: A 15-year Follow up of the Alberta Biologics Pharmacovigilance Cohort [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/switching-biologics-and-failure-to-attain-remission-in-the-first-year-predicts-bdmard-refractory-disease-in-rheumatoid-arthritis-a-15-year-follow-up-of-the-alberta-biologics-pharmacovigilance-cohort/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/switching-biologics-and-failure-to-attain-remission-in-the-first-year-predicts-bdmard-refractory-disease-in-rheumatoid-arthritis-a-15-year-follow-up-of-the-alberta-biologics-pharmacovigilance-cohort/